- Record: found
- Abstract: found
- Article: found
microRNA: The Impact on Cancer Stemness and Therapeutic Resistance
Read this article at
Abstract
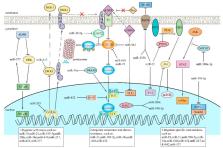
Cancer ranks as the second leading cause of death worldwide, causing a large social and economic burden. However, most anti-cancer treatments face the problems of tumor recurrence and metastasis. Therefore, finding an effective cure for cancer needs to be solved urgently. Recently, the discovery of cancer stem cells (CSCs) provides a new orientation for cancer research and therapy. CSCs share main characteristics with stem cells and are able to generate an entire tumor. Besides, CSCs usually escape from current anti-cancer therapies, which is partly responsible for tumor recurrence and poor prognosis. microRNAs (miRNAs) belong to small noncoding RNA and regulate gene post-transcriptional expression. The dysregulation of miRNAs leads to plenty of diseases, including cancer. The aberrant miRNA expression in CSCs enhances stemness maintenance. In this review, we summarize the role of miRNAs on CSCs in the eight most common cancers, hoping to bridge the research of miRNAs and CSCs with clinical applications. We found that miRNAs can act as tumor promoter or suppressor. The dysregulation of miRNAs enhances cell stemness and contributes to tumor metastasis and therapeutic resistance via the formation of feedback loops and constitutive activation of carcinogenic signaling pathways. More importantly, some miRNAs may be potential targets for diagnosis, prognosis, and cancer treatments.
Related collections
Most cited references175
- Record: found
- Abstract: found
- Article: not found
Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy.
- Record: found
- Abstract: found
- Article: not found
A protein interaction network for pluripotency of embryonic stem cells.
- Record: found
- Abstract: found
- Article: not found