- Record: found
- Abstract: found
- Article: found
PD-1, but Not PD-L1, Expressed by Islet-Reactive CD4 + T Cells Suppresses Infiltration of the Pancreas During Type 1 Diabetes
Read this article at
Abstract
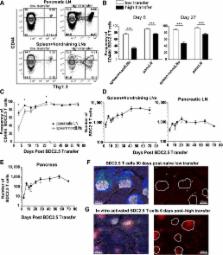
The inhibitory receptor programmed death-1 (PD-1) constrains type 1 diabetes (T1D) in the nonobese diabetic (NOD) mouse. However, how PD-1 influences diabetogenic CD4 + T cells during natural diabetes is not fully understood. To address this question, we developed a novel model to investigate antigen-specific CD4 + T cells under physiological conditions in vivo. We transferred a low number of naïve CD4 + T cells from the BDC2.5 mouse into prediabetic NOD mice to mimic a physiological precursor frequency and allowed the cells to become primed by endogenous autoantigen. Transferred BDC2.5 T cells became activated, differentiated into T-bet + IFN-γ–producing cells, and infiltrated the pancreas. In this model, loss of PD-1, but not programmed death ligand-1 (PD-L1), on the antigen-specific CD4 + T cell resulted in increased cell numbers in the spleen, pancreas-draining lymph node, and pancreas. PD-1 deficiency also increased expression of the chemokine receptor CXCR3. Lastly, histological data showed that a loss of PD-1 caused BDC2.5 cells to penetrate deep into the islet core, resulting in conversion from peri-insulitis to destructive insulitis. These data support a model by which PD-1 regulates islet-reactive CD4 + T cells in a cell intrinsic manner by suppressing proliferation, inhibiting infiltration of the pancreas, and limiting diabetes.
Related collections
Most cited references27
- Record: found
- Abstract: found
- Article: not found
Foxp3 instability leads to the generation of pathogenic memory T cells in vivo
- Record: found
- Abstract: found
- Article: not found
The Programmed Death-1 (PD-1) Pathway Regulates Autoimmune Diabetes in Nonobese Diabetic (NOD) Mice
- Record: found
- Abstract: found
- Article: not found