- Record: found
- Abstract: found
- Article: found
Zinc-Modified Titanate Nanotubes as Radiosensitizers for Glioblastoma: Enhancing Radiotherapy Efficacy and Monte Carlo Simulations
Read this article at
Abstract

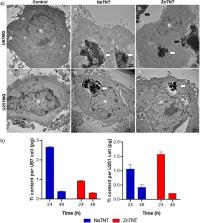
Radiotherapy (RT) is the established noninvasive treatment for glioblastoma (GBM), a highly aggressive malignancy. However, its effectiveness in improving patient survival remains limited due to the radioresistant nature of GBM. Metal-based nanostructures have emerged as promising strategies to enhance RT efficacy. Among them, titanate nanotubes (TNTs) have gained significant attention due to their biocompatibility and cost-effectiveness. This study aimed to synthesize zinc-modified TNTs (ZnTNT) from sodium TNTs (NaTNT), in addition to characterizing the formed nanostructures and evaluating their radiosensitization effects in GBM cells (U87 and U251). Hydrothermal synthesis was employed to fabricate the TNTs, which were characterized using various techniques, including transmission electron microscopy (TEM), energy-dispersive spectroscopy, scanning-transmission mode, Fourier-transform infrared spectroscopy, ICP-MS (inductively coupled plasma mass spectrometry), X-ray photoelectron spectroscopy, and zeta potential analysis. Cytotoxicity was evaluated in healthy (Vero) and GBM (U87 and U251) cells by the MTT assay, while the internalization of TNTs was observed through TEM imaging and ICP-MS. The radiosensitivity of ZnTNT and NaTNT combined with 5 Gy was evaluated using clonogenic assays. Monte Carlo simulations using the MCNP6.2 code were performed to determine the deposited dose in the culture medium for RT scenarios involving TNT clusters and cells. The results demonstrated differences in the dose deposition values between the scenarios with and without TNTs. The study revealed that ZnTNT interfered with clonogenic integrity, suggesting its potential as a powerful tool for GBM treatment.
Related collections
Most cited references123
- Record: found
- Abstract: found
- Article: not found
Principles of nanoparticle design for overcoming biological barriers to drug delivery.
- Record: found
- Abstract: found
- Article: not found
Glioma stem cells promote radioresistance by preferential activation of the DNA damage response.
- Record: found
- Abstract: found
- Article: not found