- Record: found
- Abstract: found
- Article: found
Pseudogenes and Liquid Phase Separation in Epigenetic Expression
Read this article at
Abstract
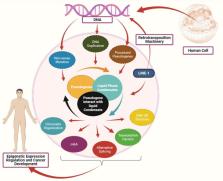
Pseudogenes have been considered as non-functional genes. However, peptides and long non-coding RNAs produced by pseudogenes are expressed in different tumors. Moreover, the dysregulation of pseudogenes is associated with cancer, and their expressions are higher in tumors compared to normal tissues. Recent studies show that pseudogenes can influence the liquid phase condensates formation. Liquid phase separation involves regulating different epigenetic stages, including transcription, chromatin organization, 3D DNA structure, splicing, and post-transcription modifications like m 6A. Several membrane-less organelles, formed through the liquid phase separate, are also involved in the epigenetic regulation, and their defects are associated with cancer development. However, the association between pseudogenes and liquid phase separation remains unrevealed. The current study sought to investigate the relationship between pseudogenes and liquid phase separation in cancer development, as well as their therapeutic implications.
Related collections
Most cited references98
- Record: found
- Abstract: found
- Article: not found
Coactivator condensation at super-enhancers links phase separation and gene control
- Record: found
- Abstract: found
- Article: not found
Organization of Chromatin by Intrinsic and Regulated Phase Separation
- Record: found
- Abstract: found
- Article: not found