- Record: found
- Abstract: found
- Article: found
Combination therapy with miR34a and doxorubicin synergistically inhibits Dox-resistant breast cancer progression via down-regulation of Snail through suppressing Notch/NF- κB and RAS/RAF/MEK/ERK signaling pathway

Read this article at
Abstract
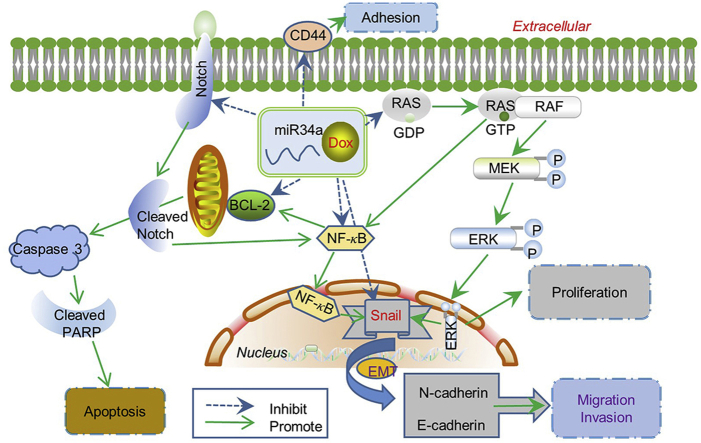
Resistance to breast cancer (BCa) chemotherapy severely hampers the patient's prognosis. MicroRNAs provide a potential therapeutic prospect for BCa. In this study, the reversal function of microRNA34a (miR34a) on doxorubicin (Dox) resistance of BCa and the possible mechanism was investigated. We found that the relative level of miR34a was significantly decreased in Dox-resistant breast cancer cell MCF-7 (MCF-7/A) compared with Dox-sensitive MCF-7 cells. Transfection with miR34a significantly suppressed the invasion, migration, adhesion of MCF-7/A cells without inhibiting their growth obviously. The combination of miR34a and Dox could significantly inhibit the proliferation, migration, invasion and induce the apoptosis of MCF-7/A cells. The synergistic effect of this combination on resistant MCF-7/A cells has no obvious relation with the expressions of classical drug-resistant proteins P-GP, MRP and GST- π, while closely related with the down-regulation on TOP2A and BCRP. Moreover, we found both protein and mRNA expression of Snail were significantly up-regulated in MCF-7/A cells in comparison with MCF-7 cells. Transfection with small interfering RNA (siRNA) of Snail could inhibit the invasion, migration and adhesion of drug-resistant MCF-7/A cells, while high-expression of Snail could remarkably promote the invasion, migration and adhesion of MCF-7 cells, which might be related with regulation of N-cadherin and E-cadherin. Transfection with miR34a in MCF-7/A cells induced a decrease of Snail expression. The potential binding sites of miR34a with 3′ UTR of Snail were predicted by miRDB target prediction software, which was confirmed by luciferase reporter gene method. Results showed that the relative activity of luciferase was reduced in MCF-7/A cells after co-transfection of miR34a and wild type (wt)-Snail, while did not change by co-transfection with miR34a and 3′ UTR mutant type (mut) Snail. Combination of miR34a and Dox induced a stronger decrease of Snail in MCF-7/A cells in comparison to miR34a or Dox treatment alone. What’ more, for the first time, we also found miR34a combined with Dox could obviously inhibit the expression of Snail through suppressing Notch/NF- κB and RAS/RAF/MEK/ERK pathway in MCF-7/A cells. In vivo study indicated that combination of miR34a and Dox significantly slowed down tumor growth in MCF-7/A nude mouse xenograft model compared with Dox alone, which was manifested by the down-regulation of Snail and pro-apoptosis effect in tumor xenografts. These results together underline the relevance of miR34a-driven regulation of Snail in drug resistance and co-administration of miR34a and Dox may produce an effective therapy outcome in the future in clinic.
Graphical abstract
Related collections
Most cited references56
- Record: found
- Abstract: found
- Article: not found
Molecular mechanisms of epithelial-mesenchymal transition.
- Record: found
- Abstract: found
- Article: not found
EMT Transition States during Tumor Progression and Metastasis
- Record: found
- Abstract: found
- Article: not found