- Record: found
- Abstract: found
- Article: found
Efficacy of sildenafil on ischaemic digital ulcer healing in systemic sclerosis: the placebo-controlled SEDUCE study
Read this article at
Abstract
Objective
To assess the effect of sildenafil, a phosphodiesterase type 5 inhibitor, on digital ulcer (DU) healing in systemic sclerosis (SSc).
Methods
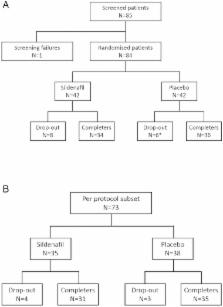
Randomised, placebo-controlled study in patients with SSc to assess the effect of sildenafil 20 mg or placebo, three times daily for 12 weeks, on ischaemic DU healing. The primary end point was the time to healing for each DU. Time to healing was compared between groups using Cox models for clustered data (two-sided tests, p=0.05).
Results
Intention-to-treat analysis involved 83 patients with a total of 192 DUs (89 in the sildenafil group and 103 in the placebo group). The HR for DU healing was 1.33 (0.88 to 2.00) (p=0.18) and 1.27 (0.85 to 1.89) (p=0.25) when adjusted for the number of DUs at entry, in favour of sildenafil. In the per protocol population, the HRs were 1.49 (0.98 to 2.28) (p=0.06) and 1.43 (0.93 to 2.19) p=0.10. The mean number of DUs per patient was lower in the sildenafil group compared with the placebo group at week (W) 8 (1.23±1.61 vs 1.79±2.40 p=0.04) and W12 (0.86±1.62 vs 1.51±2.68, p=0.01) resulting from a greater healing rate (p=0.01 at W8 and p=0.03 at W12).
Related collections
Most cited references10
- Record: found
- Abstract: found
- Article: not found
Cox regression analysis of multivariate failure time data: the marginal approach.
- Record: found
- Abstract: found
- Article: not found