- Record: found
- Abstract: found
- Article: found
Strategies for Detection of Plasmodium species Gametocytes
Read this article at
Abstract
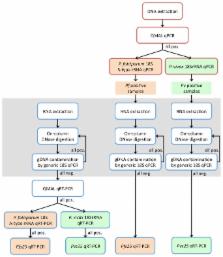
Carriage and density of gametocytes, the transmission stages of malaria parasites, are determined for predicting the infectiousness of humans to mosquitoes. This measure is used for evaluating interventions that aim at reducing malaria transmission. Gametocytes need to be detected by amplification of stage-specific transcripts, which requires RNA-preserving blood sampling. For simultaneous, highly sensitive quantification of both, blood stages and gametocytes, we have compared and optimized different strategies for field and laboratory procedures in a cross sectional survey in 315 5-9 yr old children from Papua New Guinea. qRT-PCR was performed for gametocyte markers pfs25 and pvs25, Plasmodium species prevalence was determined by targeting both, 18S rRNA genes and transcripts. RNA-based parasite detection resulted in a P. falciparum positivity of 24.1%; of these 40.8% carried gametocytes. P. vivax positivity was 38.4%, with 38.0% of these carrying gametocytes. Sensitivity of DNA-based parasite detection was substantially lower with 14.1% for P. falciparum and 19.6% for P. vivax . Using the lower DNA-based prevalence of asexual stages as a denominator increased the percentage of gametocyte-positive infections to 59.1% for P. falciparum and 52.4% for P. vivax . For studies requiring highly sensitive and simultaneous quantification of sexual and asexual parasite stages, 18S rRNA transcript-based detection saves efforts and costs. RNA-based positivity is considerably higher than other methods. On the other hand, DNA-based parasite quantification is robust and permits comparison with other globally generated molecular prevalence data. Molecular monitoring of low density asexual and sexual parasitaemia will support the evaluation of effects of up-scaled antimalarial intervention programs and can also inform about small scale spatial variability in transmission intensity.
Related collections
Most cited references28
- Record: found
- Abstract: found
- Article: not found
Improved synchronous production of Plasmodium falciparum gametocytes in vitro.

- Record: found
- Abstract: found
- Article: found
Substantial Contribution of Submicroscopical Plasmodium falciparum Gametocyte Carriage to the Infectious Reservoir in an Area of Seasonal Transmission

- Record: found
- Abstract: found
- Article: found