- Record: found
- Abstract: found
- Article: found
Staphylococcus aureus α-Toxin: Nearly a Century of Intrigue
Read this article at
Abstract
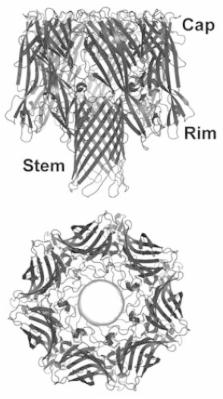
Staphylococcus aureus secretes a number of host-injurious toxins, among the most prominent of which is the small β-barrel pore-forming toxin α-hemolysin. Initially named based on its properties as a red blood cell lytic toxin, early studies suggested a far greater complexity of α-hemolysin action as nucleated cells also exhibited distinct responses to intoxication. The hemolysin, most aptly referred to as α-toxin based on its broad range of cellular specificity, has long been recognized as an important cause of injury in the context of both skin necrosis and lethal infection. The recent identification of ADAM10 as a cellular receptor for α-toxin has provided keen insight on the biology of toxin action during disease pathogenesis, demonstrating the molecular mechanisms by which the toxin causes tissue barrier disruption at host interfaces lined by epithelial or endothelial cells. This review highlights both the historical studies that laid the groundwork for nearly a century of research on α-toxin and key findings on the structural and functional biology of the toxin, in addition to discussing emerging observations that have significantly expanded our understanding of this toxin in S. aureus disease. The identification of ADAM10 as a proteinaceous receptor for the toxin not only provides a greater appreciation of truths uncovered by many historic studies, but now affords the opportunity to more extensively probe and understand the role of α-toxin in modulation of the complex interaction of S. aureus with its human host.
Related collections
Most cited references161
- Record: found
- Abstract: found
- Article: not found
Gram-positive bacteria produce membrane vesicles: proteomics-based characterization of Staphylococcus aureus-derived membrane vesicles.
- Record: found
- Abstract: not found
- Article: not found
The ADAMs family of metalloproteases: multidomain proteins with multiple functions.
- Record: found
- Abstract: found
- Article: not found