- Record: found
- Abstract: found
- Article: found
Liver, visceral and subcutaneous fat in men and women of South Asian and white European descent: a systematic review and meta-analysis of new and published data

Read this article at
Abstract
Aims/hypothesis
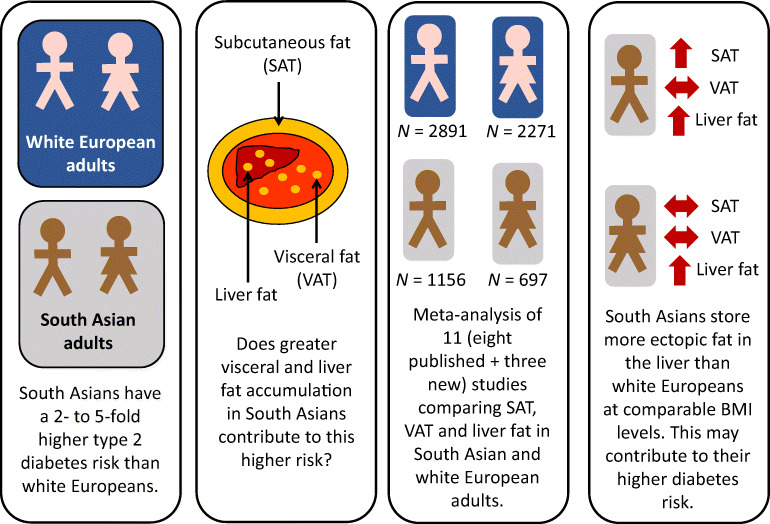
South Asians have a two- to fivefold higher risk of developing type 2 diabetes than those of white European descent. Greater central adiposity and storage of fat in deeper or ectopic depots are potential contributing mechanisms. We collated existing and new data on the amount of subcutaneous (SAT), visceral (VAT) and liver fat in adults of South Asian and white European descent to provide a robust assessment of potential ethnic differences in these factors.
Methods
We performed a systematic review of the Embase and PubMed databases from inception to August 2021. Unpublished imaging data were also included. The weighted standardised mean difference (SMD) for each adiposity measure was estimated using random-effects models. The quality of the studies was assessed using the ROBINS-E tool for risk of bias and overall certainty of the evidence was assessed using the GRADE approach. The study was pre-registered with the OSF Registries ( https://osf.io/w5bf9).
Results
We summarised imaging data on SAT, VAT and liver fat from eight published and three previously unpublished datasets, including a total of 1156 South Asian and 2891 white European men, and 697 South Asian and 2271 white European women. Despite South Asian men having a mean BMI approximately 0.5–0.7 kg/m 2 lower than white European men (depending on the comparison), nine studies showed 0.34 SMD (95% CI 0.12, 0.55; I 2=83%) more SAT and seven studies showed 0.56 SMD (95% CI 0.14, 0.98; I 2=93%) more liver fat, but nine studies had similar VAT (−0.03 SMD; 95% CI −0.24, 0.19; I 2=85%) compared with their white European counterparts. South Asian women had an approximately 0.9 kg/m 2 lower BMI but 0.31 SMD (95% CI 0.14, 0.48; I 2=53%) more liver fat than their white European counterparts in five studies. Subcutaneous fat levels (0.03 SMD; 95% CI −0.17, 0.23; I 2=72%) and VAT levels (0.04 SMD; 95% CI −0.16, 0.24; I 2=71%) did not differ significantly between ethnic groups in eight studies of women.
Conclusions/interpretation
South Asian men and women appear to store more ectopic fat in the liver compared with their white European counterparts with similar BMI levels. Given the emerging understanding of the importance of liver fat in diabetes pathogenesis, these findings help explain the greater diabetes risks in South Asians.
Related collections
Most cited references42
- Record: found
- Abstract: not found
- Article: not found
GRADE: an emerging consensus on rating quality of evidence and strength of recommendations.
- Record: found
- Abstract: found
- Article: not found