- Record: found
- Abstract: found
- Article: found
Human Cytomegalovirus-Induced Degradation of CYTIP Modulates Dendritic Cell Adhesion and Migration
Read this article at
Abstract
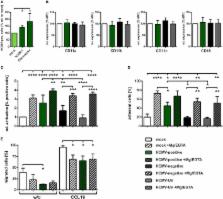
As potent antigen-presenting cells, dendritic cells (DCs) are essential for the initiation of effective antiviral immune responses. Viruses and especially herpesviruses, which are able to establish lifelong persistence, exploit several immune evasion mechanisms targeting DC biology. Our group has previously shown that the α-herpesvirus herpes simplex virus type 1 inhibits mature DC (mDC) migration by inducing adhesion via degrading the cellular protein CYTIP (cytohesin-1 interacting protein), an important negative regulator of β2-integrin activity. In the present study, we extended our analysis to the β-herpesvirus human cytomegalovirus (HCMV), to investigate whether other herpesviridae also induce such modulations. Indeed, HCMV impairs mDC transwell migration capability following a CCL19-chemokine gradient, despite equivalent expression levels of the cognate chemokine receptor CCR7 at the corresponding time points post-infection. Remarkably, HCMV infection potently induced β2-integrin activity on mDCs. Furthermore, directly HCMV-infected mDCs, exhibiting viral gene expression, strongly adhere to fibronectin and ICAM-1, in contrast to mDCs lacking infection or viral gene expression. Interestingly, HCMV-positive mDCs display a proteasome-dependent degradation of CYTIP. Contrasting the migration toward CCL19, elevated expression levels of the chemokine receptor CXCR4 in HCMV-infected mDCs were associated with functional CXCL12-chemotaxis under the herein used conditions. In summary, our results show that HCMV shapes mDC adhesion to compromise migration toward CCL19, but retaining CXCL12 responsiveness. Thus, we hypothesize that a preferred migration pattern toward the bone marrow, but not to secondary lymphoid organs, could ultimately cause a failure in the induction of potent antiviral immune responses.
Related collections
Most cited references51
- Record: found
- Abstract: found
- Article: not found
Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation
- Record: found
- Abstract: found
- Article: not found
Chemotaxis: signalling the way forward.
- Record: found
- Abstract: found
- Article: not found