- Record: found
- Abstract: found
- Article: not found
Recent Advances and Challenges of Biosensing in Point-of-Care Molecular Diagnosis
Read this article at
Abstract
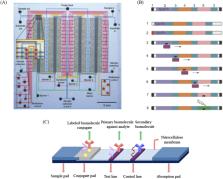
Molecular diagnosis, which plays a major role in infectious disease screening with successful understanding of the human genome, has attracted more attention because of the outbreak of COVID-19 recently. Since point-of-care testing (POCT) can expand the application of molecular diagnosis with the benefit of rapid reply, low cost, and working in decentralized environments, many researchers and commercial institutions have dedicated tremendous effort and enthusiasm to POCT-based biosensing for molecular diagnosis. In this review, we firstly summarize the state-of-the-art techniques and the construction of biosensing systems for POC molecular diagnosis. Then, the application scenarios of POCT-based biosensing for molecular diagnosis were also reviewed. Finally, several challenges and perspectives of POC biosensing for molecular diagnosis are discussed. This review is expected to help researchers deepen comprehension and make progresses in POCT-based biosensing field for molecular diagnosis applications.
Related collections
Most cited references209
- Record: found
- Abstract: found
- Article: not found
A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity.
- Record: found
- Abstract: found
- Article: not found
Multiplex genome engineering using CRISPR/Cas systems.
- Record: found
- Abstract: found
- Article: not found
