- Record: found
- Abstract: found
- Article: found
Hybrid fracture fixation systems developed for orthopaedic applications: A general review
Read this article at
Abstract
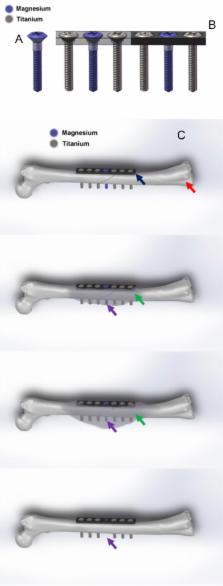
Orthopaedic implants are applied daily in our orthopaedic clinics for treatment of musculoskeletal injuries, especially for bone fracture fixation. To realise the multiple functions of orthopaedic implants, hybrid system that contains several different materials or parts have also been designed for application, such as prosthesis for total hip arthroplasty. Fixation of osteoporotic fracture is challenging as the current metal implants made of stainless steel or titanium that are rather rigid and bioinert, which are not favourable for enhancing fracture healing and subsequent remodelling. Magnesium (Mg) and its alloys are reported to possess good biocompatibility, biodegradability and osteopromotive effects during its in vivo degradation and now tested as a new generation of degradable metallic biomaterials. Several recent clinical studies reported the Mg-based screws for bone fixation, although the history of testing Mg as fixation implant was documented more than 100 years ago. Truthfully, Mg has its limitations as fixation implant, especially when applied at load-bearing sites because of rather rapid degradation. Currently developed Mg-based implants have only been designed for application at less or non–loading-bearing skeletal site(s). Therefore, after years research and development, the authors propose an innovative hybrid fixation system with parts composed of Mg and titanium or stainless steel to maximise the biological benefits of Mg; titanium or stainless steel in this hybrid system can provide enough mechanical support for fractures at load-bearing site(s) while Mg promotes the fracture healing through novel mechanisms during its degradation, especially in patients with osteoporosis and other metabolic disorders that are unfavourable conditions for fracture healing. This hybrid fixation strategy is designed to effectively enhance the osteoporotic fracture healing and may potentially also reduce the refracture rate.
The translational potential of this article: This article systemically reviewed the combination utility of different metallic implants in orthopaedic applications. It will do great contribution to the further development of internal orthopaedic implants for fracture fixation. Meanwhile, it also introduced a titanium–magnesium hybrid fixation system as an alternative fixation strategy, especially for osteoporotic patients.
Related collections
Most cited references105
- Record: found
- Abstract: found
- Article: not found
The history of biodegradable magnesium implants: a review.
- Record: found
- Abstract: found
- Article: not found
In vitro and in vivo corrosion measurements of magnesium alloys.
- Record: found
- Abstract: found
- Article: not found