- Record: found
- Abstract: found
- Article: found
Advances in All-Solid-State Lithium–Sulfur Batteries for Commercialization

Read this article at
Highlights
-
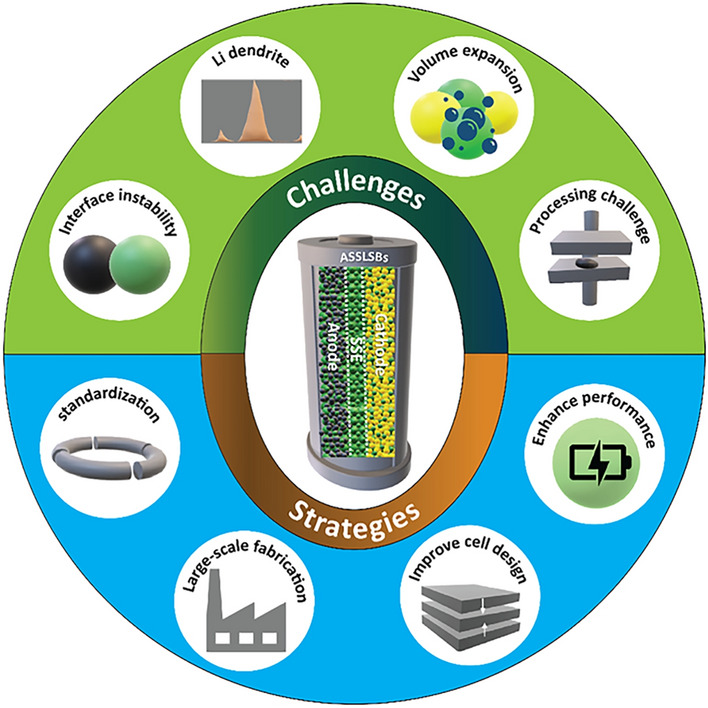
Challenges in developing practical all-solid-state lithium–sulfur batteries (ASSLSBs) and recently devised concepts to address those critical challenges have been discussed.
-
Recent developments in comprehending solid-state electrolytes, cathodes, and highperformance anodes, including key challenges associated with ion transport, electrochemical properties, and processing methods, have been discussed.
-
Prospects of ASSLSBs for commercial use and guiding forthcoming research and development efforts in this area have been presented.
Abstract
Solid-state batteries are commonly acknowledged as the forthcoming evolution in energy storage technologies. Recent development progress for these rechargeable batteries has notably accelerated their trajectory toward achieving commercial feasibility. In particular, all-solid-state lithium–sulfur batteries (ASSLSBs) that rely on lithium–sulfur reversible redox processes exhibit immense potential as an energy storage system, surpassing conventional lithium-ion batteries. This can be attributed predominantly to their exceptional energy density, extended operational lifespan, and heightened safety attributes. Despite these advantages, the adoption of ASSLSBs in the commercial sector has been sluggish. To expedite research and development in this particular area, this article provides a thorough review of the current state of ASSLSBs. We delve into an in-depth analysis of the rationale behind transitioning to ASSLSBs, explore the fundamental scientific principles involved, and provide a comprehensive evaluation of the main challenges faced by ASSLSBs. We suggest that future research in this field should prioritize plummeting the presence of inactive substances, adopting electrodes with optimum performance, minimizing interfacial resistance, and designing a scalable fabrication approach to facilitate the commercialization of ASSLSBs.
Related collections
Most cited references236
- Record: found
- Abstract: not found
- Article: not found
Reviving the lithium metal anode for high-energy batteries
- Record: found
- Abstract: found
- Article: not found
The Li-ion rechargeable battery: a perspective.
- Record: found
- Abstract: not found
- Article: not found