- Record: found
- Abstract: found
- Article: found
Combating poor-quality anti-malarial medicines: a call to action
Read this article at
Abstract
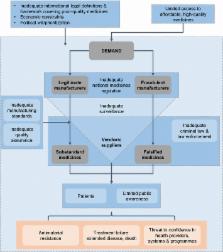
The circulation of poor-quality medicines continues to undermine the fight against many life-threatening diseases. Anti-malarial medicines appear to have been particularly compromised and present a major public health threat in malaria-endemic countries, negatively affecting individuals and their communities. Concerted collaborative efforts are required from global, regional and national organizations, involving the public and private sectors, to address the problem. While many initiatives are underway, a number of unmet needs deserve urgent and increased multisector attention. At the global level, there is a need for an international public health legal framework or treaty on poor-quality medicines, with statutes suitable for integration into national laws. In addition, increased international efforts are required to strengthen the governance of global supply chains and enhance cooperation between national medicine regulation authorities and law enforcement bodies. Increased investment is needed in innovative technologies that will enable healthcare teams to detect poor-quality medicines at all levels of the supply chain. At the regional level, a number of initiatives would be beneficial—key areas are standardization, simplification, and reciprocal recognition of registration processes and development of quality control capacity in regional centres of excellence that are better aligned with public health needs; improved surveillance methods and creation of a framework for compulsory and transparent reporting of poor-quality medicines; additional support for national medicine regulation authorities and other national partner authorities; and an increase in support for regional laboratories to boost their capabilities in detecting poor-quality medicines. It is vital that all stakeholders involved in efforts against poor-quality anti-malarial medicines extend and strengthen their actions in these critical areas and thus effectively support global health development and malaria elimination programmes.
Related collections
Most cited references39
- Record: found
- Abstract: found
- Article: not found
Poor-quality antimalarial drugs in southeast Asia and sub-Saharan Africa.
- Record: found
- Abstract: found
- Article: not found
Impact of poor-quality medicines in the ‘developing’ world

- Record: found
- Abstract: found
- Article: found
Substandard and counterfeit medicines: a systematic review of the literature
Author and article information
Comments
Comment on this article
Similar content56
- The safety of using anti-VEGF: Is there strength in numbers? Curtis LH, Hammill BG, Schulman KA, Cousins SW (2010) Risks of mortality, myocardial infarction, bleeding, and stroke associated with therapies for age-related macular degeneration. Arch Ophthalmol 128(10):1273–1279Authors: David Wong, Antonia Joussen