- Record: found
- Abstract: found
- Article: not found
The transcription factor SlWRKY37 positively regulates jasmonic acid- and dark-induced leaf senescence in tomato
Abstract
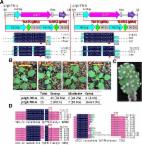
Initiation and progression of leaf senescence are triggered by various environmental stressors and phytohormones. Jasmonic acid (JA) and darkness accelerate leaf senescence in plants. However, the mechanisms that integrate these two factors to initiate and regulate leaf senescence have not been identified. Here, we report a transcriptional regulatory module centred on a novel tomato WRKY transcription factor, SlWRKY37, responsible for both JA- and dark-induced leaf senescence. The expression of SlWRKY37, together with SlMYC2, encoding a master transcription factor in JA signalling, was significantly induced by both methyl jasmonate (MeJA) and dark treatments. SlMYC2 binds directly to the promoter of SlWRKY37 to activate its expression. Knock out of SlWRKY37 inhibited JA- and dark-induced leaf senescence. Transcriptome analysis and biochemical experiments revealed SlWRKY53 and SlSGR1 (S. lycopersicum senescence-inducible chloroplast stay-green protein 1) as direct transcriptional targets of SlWRKY37 to control leaf senescence. Moreover, SlWRKY37 interacted with a VQ motif-containing protein SlVQ7, and the interaction improved the stability of SlWRKY37 and the transcriptional activation of downstream target genes. Our results reveal the physiological and molecular functions of SlWRKY37 in leaf senescence, and offer a target gene to retard leaf yellowing by reducing sensitivity to external senescence signals, such as JA and darkness.
Related collections
Most cited references84
- Record: found
- Abstract: found
- Article: not found
Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis.
- Record: found
- Abstract: found
- Article: found
A CRISPR/Cas9 toolkit for multiplex genome editing in plants
- Record: found
- Abstract: found
- Article: not found