- Record: found
- Abstract: found
- Article: found
Sodium butyrate alleviates free fatty acid-induced steatosis in primary chicken hepatocytes via the AMPK/PPARα pathway
Read this article at
Abstract
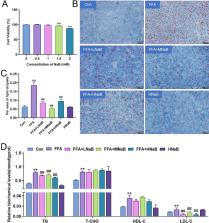
Fatty liver hemorrhagic syndrome ( FLHS) is a prevalent metabolic disorder observed in egg-laying hens, characterized by fatty deposits and cellular steatosis in the liver. Our preliminary investigations have revealed a marked decrease in the concentration of butyric acid in the FLHS strain of laying hens. It has been established that sodium butyrate ( NaB) protects against metabolic disorders. However, the underlying mechanism by which butyrate modulates hepato-lipid metabolism to a great extent remains unexplored. In this study, we constructed an isolated in vitro model of chicken primary hepatocytes to induce hepatic steatosis by free fatty acids ( FFA). Our results demonstrate that treatment with NaB effectively mitigated FFA-induced hepatic steatosis in chicken hepatocytes by inhibiting lipid accumulation, downregulating the mRNA expression of lipo-synthesis-related genes (sterol regulatory element binding transcription factor 1 ( SREBF1 ), acetyl-CoA carboxylase 1( ACC1 ), fatty acid synthase ( FASN ), stearoyl-CoA desaturase 1 ( SCD1 ), liver X receptor α ( LXRα ), 3-hydroxy-3-methylglutaryl-CoA reductase ( HMGR )) ( P < 0.05), and upregulating the mRNA and protein expression of AMP-activated protein kinase α1 ( AMPKα1), peroxisome proliferator-activated receptor α ( PPARα), and carnitine palmitoyl-transferase 1A ( CPT1A) ( P < 0.05). Moreover, AMPK and PPARα inhibitors (Compound C ( Comp C) and GW6471, respectively) reversed the protective effects of NaB against FFA-induced hepatic steatosis by blocking the AMPK/PPARα pathway, leading to lipid droplet accumulation and triglyceride ( TG) contents in chicken primary hepatocytes. With these findings, NaB can alleviate hepatocyte lipoatrophy injury by activating the AMPK/PPARα pathway, promoting fatty acid oxidation, and reducing lipid synthesis in chicken hepatocytes, potentially being able to provide new ideas for the treatment of FLHS.
Related collections
Most cited references46
- Record: found
- Abstract: found
- Article: not found
A metagenome-wide association study of gut microbiota in type 2 diabetes.
- Record: found
- Abstract: found
- Article: not found
The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon.
- Record: found
- Abstract: found
- Article: not found