- Record: found
- Abstract: found
- Article: found
ZBP1 governs the inflammasome-independent IL-1α and neutrophil inflammation that play a dual role in anti-influenza virus immunity
Read this article at
Abstract
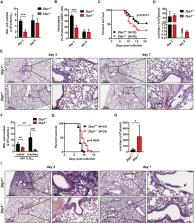
Influenza A virus (IAV) triggers the infected lung to produce IL-1 and recruit neutrophils. Unlike IL-1β, however, little is known about IL-1α in terms of its mechanism of induction, action and physiological relevance to the host immunity against IAV infection. In particular, whether Z-DNA-binding protein 1 (ZBP1), a key molecule for IAV-induced cell death, is involved in the IL-1α induction, neutrophil infiltration and the physiological outcome has not been elucidated. Here, we show in a murine model that the IAV-induced IL-1α is mediated solely by ZBP1, in an NLRP3-inflammasome-independent manner, and is required for the optimal IL-1β production followed by the formation of neutrophil extracellular traps (NETs). During IAV infection, ZBP1 displays a dual role in anti-IAV immune responses mediated by neutrophils, resulting in either protective or pathological outcomes in vivo. Thus, ZBP1-mediated IL-1α production is the key initial step of IAV-infected NETs, regulating the duality of the consequent lung inflammation.
Abstract
ZBP1 and IL-1 control lung inflammation in influenza
Related collections
Most cited references53
- Record: found
- Abstract: found
- Article: not found
Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression.

- Record: found
- Abstract: found
- Article: found
Neutrophil Extracellular Traps Directly Induce Epithelial and Endothelial Cell Death: A Predominant Role of Histones
- Record: found
- Abstract: found
- Article: not found