- Record: found
- Abstract: found
- Article: found
The gamble between oncolytic virus therapy and IFN
Read this article at
Abstract
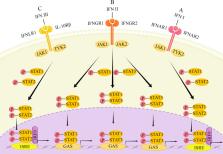
Various studies are being conducted on oncolytic virotherapy which one of the mechanisms is mediating interferon (IFN) production by it exerts antitumor effects. The antiviral effect of IFN itself has a negative impact on the inhibition of oncolytic virus or tumor eradication. Therefore, it is very critical to understand the mechanism of IFN regulation by oncolytic viruses, and to define its mechanism is of great significance for improving the antitumor effect of oncolytic viruses. This review focuses on the regulatory mechanisms of IFNs by various oncolytic viruses and their combination therapies. In addition, the exerting and the producing pathways of IFNs are briefly summarized, and some current issues are put forward.
Related collections
Most cited references171
- Record: found
- Abstract: found
- Article: not found
Nivolumab versus Docetaxel in Advanced Nonsquamous Non–Small-Cell Lung Cancer
- Record: found
- Abstract: found
- Article: not found
PD-1 blockade induces responses by inhibiting adaptive immune resistance
- Record: found
- Abstract: found
- Article: not found