- Record: found
- Abstract: found
- Article: found
Unveiling a novel memory center in human brain: neurochemical identification of the nucleus incertus, a key pontine locus implicated in stress and neuropathology
Read this article at
Abstract
Background
The nucleus incertus (NI) was originally described by Streeter in 1903, as a midline region in the floor of the fourth ventricle of the human brain with an ‘unknown’ function. More than a century later, the neuroanatomy of the NI has been described in lower vertebrates, but not in humans. Therefore, we examined the neurochemical anatomy of the human NI using markers, including the neuropeptide, relaxin-3 (RLN3), and began to explore the distribution of the NI-related RLN3 innervation of the hippocampus.
Methods
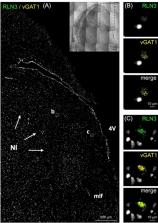
Histochemical staining of serial, coronal sections of control human postmortem pons was conducted to reveal the presence of the NI by detection of immunoreactivity (IR) for the neuronal markers, microtubule-associated protein-2 (MAP2), glutamic acid dehydrogenase (GAD)-65/67 and corticotrophin-releasing hormone receptor 1 (CRHR1), and RLN3, which is highly expressed in NI neurons in diverse species. RLN3 and vesicular GABA transporter 1 ( vGAT1) mRNA were detected by fluorescent in situ hybridization. Pons sections containing the NI from an AD case were immunostained for phosphorylated-tau, to explore potential relevance to neurodegenerative diseases. Lastly, sections of the human hippocampus were stained to detect RLN3-IR and somatostatin (SST)-IR.
Results
In the dorsal, anterior-medial region of the human pons, neurons containing RLN3- and MAP2-IR, and RLN3/vGAT1 mRNA-positive neurons were observed in an anatomical pattern consistent with that of the NI in other species. GAD65/67- and CRHR1-immunopositive neurons were also detected within this area. Furthermore, RLN3- and AT8-IR were co-localized within NI neurons of an AD subject. Lastly, RLN3-IR was detected in neurons within the CA1, CA2, CA3 and DG areas of the hippocampus, in the absence of RLN3 mRNA. In the DG, RLN3- and SST-IR were co-localized in a small population of neurons.
Conclusions
Aspects of the anatomy of the human NI are shared across species, including a population of stress-responsive, RLN3-expressing neurons and a RLN3 innervation of the hippocampus. Accumulation of phosphorylated-tau in the NI suggests its possible involvement in AD pathology. Further characterization of the neurochemistry of the human NI will increase our understanding of its functional role in health and disease.
Related collections
Most cited references93
- Record: found
- Abstract: found
- Article: not found
Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years.
- Record: found
- Abstract: found
- Article: not found
