- Record: found
- Abstract: found
- Article: found
The Glycan Shield of HIV Is Predominantly Oligomannose Independently of Production System or Viral Clade
Read this article at
Abstract
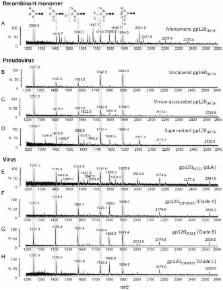
The N-linked oligomannose glycans of HIV gp120 are a target for both microbicide and vaccine design. The extent of cross-clade conservation of HIV oligomannose glycans is therefore a critical consideration for the development of HIV prophylaxes. We measured the oligomannose content of virion-associated gp120 from primary virus from PBMCs for a range of viral isolates and showed cross-clade elevation (62–79%) of these glycans relative to recombinant, monomeric gp120 (∼30%). We also confirmed that pseudoviral production systems can give rise to notably elevated gp120 oligomannose levels (∼98%), compared to gp120 derived from a single-plasmid viral system using the HIV LAI backbone (56%). This study highlights differences in glycosylation between virion-associated and recombinant gp120.
Related collections
Most cited references41
- Record: found
- Abstract: not found
- Article: not found
Assembly of asparagine-linked oligosaccharides.
- Record: found
- Abstract: not found
- Article: not found
HIV vaccine design and the neutralizing antibody problem.
- Record: found
- Abstract: found
- Article: not found