- Record: found
- Abstract: found
- Article: not found
Melanoma risk and survival among organ transplant recipients
Read this article at
Abstract
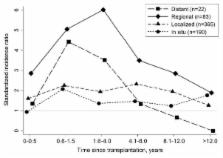
Solid organ transplant recipients, who are medically immunosuppressed to prevent graft rejection, have increased melanoma risk, but risk factors and outcomes are incompletely documented. We evaluated melanoma incidence among 139,991 non-Hispanic white transplants using linked U.S. transplant-cancer registry data (1987–2010). We used standardized incidence ratios (SIRs) to compare incidence to the general population, and incidence rate ratios (IRRs) from multivariable Poisson models to assess risk factors. Separately, we compared post-melanoma survival among transplant recipients (N=182) and non-recipients (N=131,358) using multivariable Cox models. Among transplant recipients, risk of invasive melanoma (N=519) was elevated (SIR=2.20, 95%CI 2.01-2.39), especially for regional stage tumors (SIR=4.11, 95%CI 3.27–5.09). Risk of localized tumors was stable over time after transplantation, but higher with azathioprine maintenance therapy (IRR=1.35, 95%CI 1.03–1.77). Risk of regional/distant stage tumors peaked within 4 years following transplantation and increased with polyclonal antibody induction therapy (IRR=1.65, 95%CI 1.02–2.67). Melanoma-specific mortality was higher among transplant recipients than non-recipients (HR 2.98, 95%CI 2.26–3.93). Melanoma exhibits increased incidence and aggressive behavior under transplant-related immunosuppression. Some localized melanomas may result from azathioprine, which acts synergistically with ultraviolet radiation, while T-cell depleting induction therapies may promote late stage tumors. Our findings support sun safety practices and skin screening for transplant recipients.
Related collections
Most cited references42
- Record: found
- Abstract: found
- Article: not found
Tumor-infiltrating lymphocyte grade is an independent predictor of sentinel lymph node status and survival in patients with cutaneous melanoma.
- Record: found
- Abstract: found
- Article: not found
Cancer after kidney transplantation in the United States.
- Record: found
- Abstract: found
- Article: not found
