- Record: found
- Abstract: found
- Article: found
miR-145 inhibits tumor growth and metastasis by targeting metadherin in high-grade serous ovarian carcinoma
Read this article at
Abstract
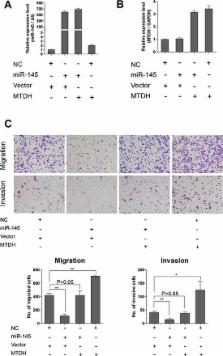
High-grade serous ovarian carcinoma (HGSOC), the most common and aggressive subtype of epithelial ovarian cancer, is characterized by TP53 mutations and genetic instability. Using miRNA profiling analysis, we found that miR-145, a p53 regulated miRNA, was frequently down-regulated in HGSOC. miR-145 down-regulation was further validated in a large cohort of HGSOCs by qPCR. Overexpression of miR-145 in ovarian cancer cells significantly suppressed proliferation, migration and invasion in vitro and inhibited tumor growth and metastasis in vivo. Metadherin (MTDH) was subsequently identified as a direct target of miR-145, and was found to be significantly up-regulated in HGSOC. Furthermore, overexpression of MTDH rescued the inhibitory effects of miR-145 in ovarian cancer cells. Finally, we found that high level of MTDH expression correlated with poor prognosis of HGSOC. Therefore, lack of suppression of MTDH by miR-145 when p53 is dysfunctional leads to increased tumor growth and metastasis of HGSOC. Our study established a new link between p53, miR-145 and MTDH in the regulation of tumor growth and metastasis in HGSOC.
Related collections
Most cited references21
- Record: found
- Abstract: found
- Article: not found
miR-145 and miR-143 Regulate Smooth Muscle Cell Fate Decisions
- Record: found
- Abstract: found
- Article: not found
MicroRNA expression profiles in serous ovarian carcinoma.
- Record: found
- Abstract: found
- Article: not found