- Record: found
- Abstract: found
- Article: found
The inhibition of fibril formation of lysozyme by sucrose and trehalose†

Read this article at
Abstract
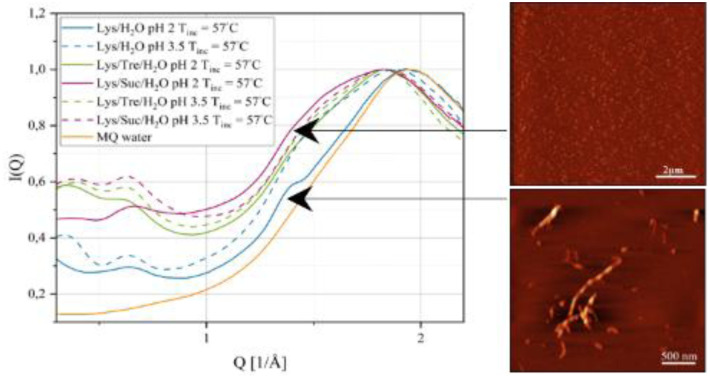
The two disaccharides, trehalose and sucrose, have been compared in many studies due to their structural similarity. Both possess the ability to stabilise and reduce aggregation of proteins. Trehalose has also been shown to inhibit the formation of highly structured protein aggregates called amyloid fibrils. This study aims to compare how the thermal stability of the protein lysozyme at low pH (2.0 and 3.5) is affected by the presence of the two disaccharides. We also address the anti-aggregating properties of the disaccharides and their inhibitory effects on fibril formation. Differential scanning calorimetry confirms that the thermal stability of lysozyme is increased by the presence of trehalose or sucrose. The effect is slightly larger for sucrose. The inhibiting effects on protein aggregation are investigated using small-angle X-ray scattering which shows that the two-component system consisting of lysozyme and water (Lys/H 2O) at pH 2.0 contains larger aggregates than the corresponding system at pH 3.5 as well as the sugar containing systems. In addition, the results show that the particle-to-particle distance in the sugar containing systems (Lys/Tre/H 2O and Lys/Suc/H 2O) at pH 2.0 is longer than at pH 3.5, suggesting larger protein aggregates in the former. Finally, the characteristic distance separating β-strands in amyloid fibrils is observed for the Lys/H 2O system at pH 2.0, using wide-angle X-ray scattering, while it is not clearly observed for the sugar containing systems. This study further shows that the two disaccharides stabilise the native fold of lysozyme by increasing the denaturation temperature. However, other factors, such as a weakening of hydrophobic interactions and hydrogen bonding between proteins, might also play a role in their inhibitory effect on amyloid fibril formation.
Abstract
WAXS displays a significant difference due to the presence of amyloid fibrils in the absence of sugar.
Related collections
Most cited references3
- Record: found
- Abstract: not found
- Article: not found
Gwyddion: an open-source software for SPM data analysis
- Record: found
- Abstract: not found
- Book Chapter: not found
Biotechnological Applications of the Disaccharide Trehalose
- Record: found
- Abstract: not found
- Book: not found