- Record: found
- Abstract: found
- Article: found
Mast Cells and Their Progenitors in Allergic Asthma
Read this article at
Abstract
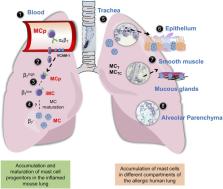
Mast cells and their mediators have been implicated in the pathogenesis of asthma and allergy for decades. Allergic asthma is a complex chronic lung disease in which several different immune cells, genetic factors and environmental exposures influence the pathology. Mast cells are key players in the asthmatic response through secretion of a multitude of mediators with pro-inflammatory and airway-constrictive effects. Well-known mast cell mediators, such as histamine and bioactive lipids are responsible for many of the physiological effects observed in the acute phase of allergic reactions. The accumulation of mast cells at particular sites of the allergic lung is likely relevant to the asthma phenotype, severity and progression. Mast cells located in different compartments in the lung and airways have different characteristics and express different mediators. According to in vivo experiments in mice, lung mast cells develop from mast cell progenitors induced by inflammatory stimuli to migrate to the airways. Human mast cell progenitors have been identified in the blood circulation. A high frequency of circulating human mast cell progenitors may reflect ongoing pathological changes in the allergic lung. In allergic asthma, mast cells become activated mainly via IgE-mediated crosslinking of the high affinity receptor for IgE (FcεRI) with allergens. However, mast cells can also be activated by numerous other stimuli e.g. toll-like receptors and MAS-related G protein-coupled receptor X2. In this review, we summarize research with implications on the role and development of mast cells and their progenitors in allergic asthma and cover selected activation pathways and mast cell mediators that have been implicated in the pathogenesis. The review places an emphasis on describing mechanisms identified using in vivo mouse models and data obtained by analysis of clinical samples.
Related collections
Most cited references289
- Record: found
- Abstract: found
- Article: not found
Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161.
- Record: found
- Abstract: found
- Article: not found
Identification of a mast cell specific receptor crucial for pseudo-allergic drug reactions
- Record: found
- Abstract: found
- Article: not found