- Record: found
- Abstract: found
- Article: found
Transferrin-targeted porous silicon nanoparticles reduce glioblastoma cell migration across tight extracellular space
Abstract
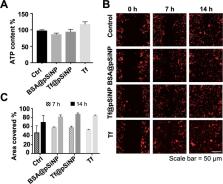
Mortality of glioblastoma multiforme (GBM) has not improved over the last two decades despite medical breakthroughs in the treatment of other types of cancers. Nanoparticles hold tremendous promise to overcome the pharmacokinetic challenges and off-target adverse effects. However, an inhibitory effect of nanoparticles by themselves on metastasis has not been explored. In this study, we developed transferrin-conjugated porous silicon nanoparticles (Tf@pSiNP) and studied their effect on inhibiting GBM migration by means of a microfluidic-based migration chip. This platform, designed to mimic the tight extracellular migration tracts in brain parenchyma, allowed high-content time-resolved imaging of cell migration. Tf@pSiNP were colloidally stable, biocompatible, and their uptake into GBM cells was enhanced by receptor-mediated internalisation. The migration of Tf@pSiNP-exposed cells across the confined microchannels was suppressed, but unconfined migration was unaffected. The pSiNP-induced destabilisation of focal adhesions at the leading front may partially explain the migration inhibition. More corroborating evidence suggests that pSiNP uptake reduced the plasticity of GBM cells in reducing cell volume, an effect that proved crucial in facilitating migration across the tight confined tracts. We believe that the inhibitory effect of Tf@pSiNP on cell migration, together with the drug-delivery capability of pSiNP, could potentially offer a disruptive strategy to treat GBM.
Related collections
Most cited references71
- Record: found
- Abstract: found
- Article: found
The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary.
- Record: found
- Abstract: found
- Article: not found
Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma
- Record: found
- Abstract: found
- Article: not found