- Record: found
- Abstract: found
- Article: found
Comparison of methods for the analysis of therapeutic immunoglobulin G Fc-glycosylation profiles—Part 1: Separation-based methods
Read this article at
Abstract
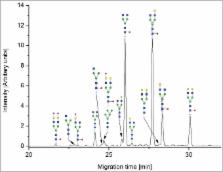
Immunoglobulin G (IgG) crystallizable fragment (Fc) glycosylation is crucial for antibody effector functions, such as antibody-dependent cell-mediated cytotoxicity, and for their pharmacokinetic and pharmacodynamics behavior. To monitor the Fc-glycosylation in bioprocess development, as well as product characterization and release analytics, reliable techniques for glycosylation analysis are needed. A wide range of analytical methods has found its way into these applications. In this study, a comprehensive comparison was performed of separation-based methods for Fc-glycosylation profiling of an IgG biopharmaceutical. A therapeutic antibody reference material was analyzed 6-fold on 2 different days, and the methods were compared for precision, accuracy, throughput and other features; special emphasis was placed on the detection of sialic acid-containing glycans. Seven, non-mass spectrometric methods were compared; the methods utilized liquid chromatography-based separation of fluorescent-labeled glycans, capillary electrophoresis-based separation of fluorescent-labeled glycans, or high-performance anion exchange chromatography with pulsed amperometric detection. Hydrophilic interaction liquid chromatography-ultra high performance liquid chromatography of 2-aminobenzamide (2-AB)-labeled glycans was used as a reference method. All of the methods showed excellent precision and accuracy; some differences were observed, particularly with regard to the detection and quantitation of minor glycan species, such as sialylated glycans.
Related collections
Most cited references60
- Record: found
- Abstract: found
- Article: not found
Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG.
- Record: found
- Abstract: found
- Article: not found
Intravenous gammaglobulin suppresses inflammation through a novel T(H)2 pathway.
- Record: found
- Abstract: found
- Article: not found