- Record: found
- Abstract: found
- Article: found
Identification of catalytic sites for oxygen reduction and oxygen evolution in N-doped graphene materials: Development of highly efficient metal-free bifunctional electrocatalyst
Read this article at
Abstract
Doping of graphene with nitrogen imparted bifunctional electrocatalytic activities for efficient energy conversion and storage.
Abstract
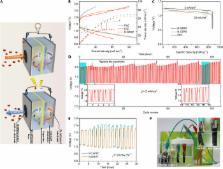
Oxygen reduction reaction (ORR) and oxygen evolution reaction (OER) are critical to renewable energy conversion and storage technologies. Heteroatom-doped carbon nanomaterials have been reported to be efficient metal-free electrocatalysts for ORR in fuel cells for energy conversion, as well as ORR and OER in metal-air batteries for energy storage. We reported that metal-free three-dimensional (3D) graphene nanoribbon networks (N-GRW) doped with nitrogen exhibited superb bifunctional electrocatalytic activities for both ORR and OER, with an excellent stability in alkaline electrolytes (for example, KOH). For the first time, it was experimentally demonstrated that the electron-donating quaternary N sites were responsible for ORR, whereas the electron-withdrawing pyridinic N moieties in N-GRW served as active sites for OER. The unique 3D nanoarchitecture provided a high density of the ORR and OER active sites and facilitated the electrolyte and electron transports. As a result, the as-prepared N-GRW holds great potential as a low-cost, highly efficient air cathode in rechargeable metal-air batteries. Rechargeable zinc-air batteries with the N-GRW air electrode in a two-electrode configuration exhibited an open-circuit voltage of 1.46 V, a specific capacity of 873 mAh g −1, and a peak power density of 65 mW cm −2, which could be continuously charged and discharged with an excellent cycling stability. Our work should open up new avenues for the development of various carbon-based metal-free bifunctional electrocatalysts of practical significance.
Related collections
Most cited references28
- Record: found
- Abstract: found
- Article: not found
High-performance electrocatalysts for oxygen reduction derived from polyaniline, iron, and cobalt.
- Record: found
- Abstract: found
- Article: not found
Co3O4 nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction
- Record: found
- Abstract: found
- Article: not found