- Record: found
- Abstract: found
- Article: found
CAR-T lymphocyte-based cell therapies; mechanistic substantiation, applications and biosafety enhancement with suicide genes: new opportunities to melt side effects
Read this article at
Abstract
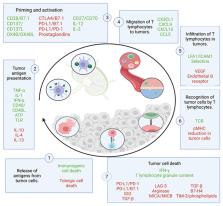
Immunotherapy has made significant strides in cancer treatment with strategies like checkpoint blockade antibodies and adoptive T cell transfer. Chimeric antigen receptor T cells (CAR-T) have emerged as a promising approach to combine these strategies and overcome their limitations. This review explores CAR-T cells as a living drug for cancer treatment. CAR-T cells are genetically engineered immune cells designed to target and eliminate tumor cells by recognizing specific antigens. The study involves a comprehensive literature review on CAR-T cell technology, covering structure optimization, generations, manufacturing processes, and gene therapy strategies. It examines CAR-T therapy in haematologic cancers and solid tumors, highlighting challenges and proposing a suicide gene-based mechanism to enhance safety. The results show significant advancements in CAR-T technology, particularly in structure optimization and generation. The manufacturing process has improved for broader clinical application. However, a series of inherent challenges and side effects still need to be addressed. In conclusion, CAR-T cells hold great promise for cancer treatment, but ongoing research is crucial to improve efficacy and safety for oncology patients. The proposed suicide gene-based mechanism offers a potential solution to mitigate side effects including cytokine release syndrome (the most common toxic side effect of CAR-T therapy) and the associated neurotoxicity.
Related collections
Most cited references180
- Record: found
- Abstract: found
- Article: not found
Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries
- Record: found
- Abstract: found
- Article: not found