- Record: found
- Abstract: found
- Article: not found
The FOXD1 lineage of kidney perivascular cells and myofibroblasts: functions and responses to injury
Read this article at
Abstract
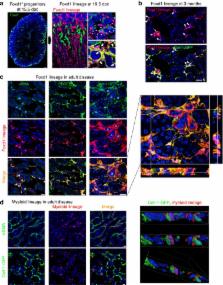
Recent studies have identified a poorly appreciated yet extensive population of perivascular mesenchymal cells in the kidney, which are derived from metanephric mesenchyme progenitor cells during nephrogenesis at which time they express the transcription factor FOXD1. Some studies have called these resident fibroblasts, whereas others have called them pericytes. Regardless of nomenclature, many are partially integrated into the capillary basement membrane and contribute in important ways to the homeostasis of peritubular capillaries. Fate-mapping studies using conditional CreER recombinase-mediated tracing of discrete cell cohorts have identified these pericytes and resident fibroblasts as the major precursor population of interstitial myofibroblasts in animal models of kidney disease. Here, we will review the evidence that they are the major population of myofibroblast precursors, highlight some critical functions in homeostasis, and focus on the cell signaling pathways that are important to their differentiation into, and persistence as myofibroblasts.
Related collections
Most cited references44
- Record: found
- Abstract: found
- Article: not found
Conditional gene targeting in macrophages and granulocytes using LysMcre mice.
- Record: found
- Abstract: found
- Article: not found
Origin and function of myofibroblasts in kidney fibrosis.
- Record: found
- Abstract: found
- Article: not found
