- Record: found
- Abstract: found
- Article: found
Senolytics reduce coronavirus-related mortality in old mice

Read this article at
Abstract
INTRODUCTION:
The COVID-19 pandemic revealed enhanced vulnerability of the elderly and chronically ill to adverse outcomes upon severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Senescence is a cell fate elicited by cellular stress that results in changes in gene expression, morphology, metabolism, and resistance to apoptosis. Senescent cells (SnCs) secrete pro-inflammatory factors, called the senescence-associated secretory phenotype (SASP). SnCs accumulate with age and drive chronic inflammation. In human cells and tissues and using a new infection paradigm, we asked whether SnCs are a cause of adverse outcomes of infection with aging. This is relevant because SnCs can be selectively eliminated in vivo with a new class of therapeutics called senolytics, potentially affording a new approach to treat COVID-19.
RATIONALE:
We hypothesized that SnCs, because of their pro-inflammatory SASP, might have a heightened response to pathogen-associated molecular pattern (PAMP) factors, resulting in increased risk of cytokine storm and multi-organ failure. To test this, we treated senescent and nonsenescent human cells with the PAMPs lipopolysaccharide (LPS) and SARS-CoV-2 spike protein (S1) and measured the SASP and its effect on non-SnCs. Similarly, old and progeroid mice were challenged with LPS, and we measured the SASP. Previously, we created a “normal microbial experience” (NME) for mice by transmitting environmental pathogens to specified-pathogen–free (SPF) mice through exposure to pet store mice or their bedding. The first pathogen transferred was mouse hepatitis virus (MHV), a β-coronavirus closely related to SARS-CoV-2. NME rapidly killed aged SPF mice known to have an increased burden of SnCs compared with young SPF mice, which survive NME. This afforded an experimental paradigm to test whether senolytics blunt adverse outcomes in β-coronavirus infection.
RESULTS:
Human endothelial SnCs became hyperinflammatory in response to challenge with LPS and S1, relative to non-SnCs. The PAMP-elicited secretome of SnCs caused increased expression of viral entry proteins and reduced expression of antiviral genes in non-senescent human endothelial and lung epithelial cells, and the proximity of these events was established in human lung biopsies. Treatment of old mice with LPS significantly increased SASP expression in several organs relative to young mice, confirming our hypothesis in vivo. Similarly, old mice exposed to NME displayed a significant multi-organ increase in SnCs and the SASP, impaired immune response to MHV, and 100% mortality, whereas inoculation with antibodies against MHV before NME afforded complete rescue of mortality. Treating old mice with the senolytic fisetin, which selectively eliminates SnCs after NME reduced mortality by 50%, reduced expression of inflammatory proteins in serum and tissue and improved the immune response. This was confirmed with a second senolytic regimen, Dasatinib plus Quercetin, as well as genetic ablation of SnCs in aged mice, establishing SnCs as a cause of adverse outcomes in aged organisms exposed to a new viral pathogen.
CONCLUSION:
SnCs amplify susceptibility to COVID-19 and pathogen-induced hyperinflammation. Reducing SnC burden in aged mice reduces mortality after pathogen exposure, including a β-coronavirus. Our findings strongly support the Geroscience hypothesis that therapeutically targeting fundamental aging mechanisms improves resilience in the elderly, with alleviation of morbidity and mortality due to pathogenic stress. This suggests that senolytics might protect others vulnerable to adverse COVID-19 outcomes in whom increased SnCs occur (such as in obesity or numerous chronic diseases).
Graphical Abstract
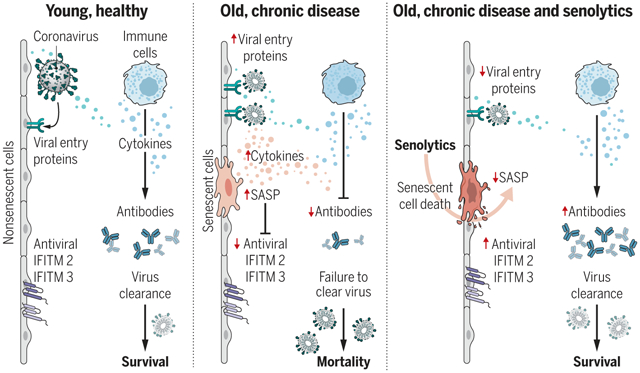
SnCs that accumulate with age or chronic disease react to PAMPs such as SARS-CoV-2 S1 by amplifying the SASP, which increases viral entry protein expression and decreases viral defense IFITMs in normal cells. Old mice exposed to pathogens such as the β-coronavirus MHV have increased inflammation and higher mortality. Treatment with a senolytic decreased SnCs, inflammation, and mortality and increased the antiviral antibody response.
Abstract
The COVID-19 pandemic has revealed the pronounced vulnerability of the elderly and chronically ill to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)–induced morbidity and mortality. Cellular senescence contributes to inflammation, multiple chronic diseases, and age-related dysfunction, but effects on responses to viral infection are unclear. Here, we demonstrate that senescent cells (SnCs) become hyper-inflammatory in response to pathogen-associated molecular patterns (PAMPs), including SARS-CoV-2 spike protein-1, increasing expression of viral entry proteins and reducing antiviral gene expression in non-SnCs through a paracrine mechanism. Old mice acutely infected with pathogens that included a SARS-CoV-2–related mouse β-coronavirus experienced increased senescence and inflammation, with nearly 100% mortality. Targeting SnCs by using senolytic drugs before or after pathogen exposure significantly reduced mortality, cellular senescence, and inflammatory markers and increased antiviral antibodies. Thus, reducing the SnC burden in diseased or aged individuals should enhance resilience and reduce mortality after viral infection, including that of SARS-CoV-2.
Related collections
Most cited references59
- Record: found
- Abstract: found
- Article: not found
Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China
- Record: found
- Abstract: found
- Article: found
COVID-19: consider cytokine storm syndromes and immunosuppression
- Record: found
- Abstract: found
- Article: not found