- Record: found
- Abstract: found
- Article: not found
Early peripheral blood MCEMP1 and HLA-DRA expression predicts COVID-19 prognosis
Read this article at
Abstract
Background
Mass vaccination has dramatically reduced the incidence of severe COVID-19, with most cases now presenting as self-limiting upper respiratory tract infections. However, those with co-morbidities, the elderly and immunocompromised, as well as the unvaccinated, remain disproportionately vulnerable to severe COVID-19 and its sequelae. Furthermore, as the effectiveness of vaccination wanes with time, immune escape SARS-CoV-2 variants could emerge to cause severe COVID-19. Reliable prognostic biomarkers for severe disease could be used as early indicator of re-emergence of severe COVID-19 as well as for triaging of patients for antiviral therapy.
Methods
We performed a systematic review and re-analysis of 7 publicly available datasets, analysing a total of 140 severe and 181 mild COVID-19 patients, to determine the most consistent differentially regulated genes in peripheral blood of severe COVID-19 patients. In addition, we included an independent cohort where blood transcriptomics of COVID-19 patients were prospectively and longitudinally monitored previously, to track the time in which these gene expression changes occur before nadir of respiratory function. Single cell RNA-sequencing of peripheral blood mononuclear cells from publicly available datasets was then used to determine the immune cell subsets involved.
Findings
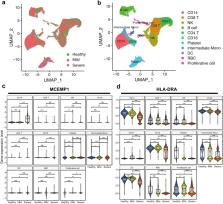
The most consistent differentially regulated genes in peripheral blood of severe COVID-19 patients were MCEMP1, HLA-DRA and ETS1 across the 7 transcriptomics datasets. Moreover, we found significantly heightened MCEMP1 and reduced HLA-DRA expression as early as four days before the nadir of respiratory function, and the differential expression of MCEMP1 and HLA-DRA occurred predominantly in CD14+ cells. The online platform which we developed is publicly available at https://kuanrongchan-covid19-severity-app-t7l38g.streamlitapp.com/, for users to query gene expression differences between severe and mild COVID-19 patients in these datasets.
Interpretation
Elevated MCEMP1 and reduced HLA-DRA gene expression in CD14+ cells during the early phase of disease are prognostic of severe COVID-19.
Funding
K.R.C is funded by the doi 10.13039/501100001349, National Medical Research Council; (NMRC) of Singapore under the Open Fund Individual Research Grant (MOH-000610). E.E.O. is funded by the doi 10.13039/501100001349, NMRC; Senior Clinician-Scientist Award (MOH-000135-00). J.G.H.L. is funded by the doi 10.13039/501100001349, NMRC; under the Clinician-Scientist Award (NMRC/CSAINV/013/2016-01). S.K. is funded by the doi 10.13039/501100001349, NMRC; under the Transition Award. This study was sponsored in part by a generous gift from The Hour Glass.
Related collections
Most cited references59
- Record: found
- Abstract: found
- Article: not found
Integrating single-cell transcriptomic data across different conditions, technologies, and species
- Record: found
- Abstract: found
- Article: not found
Clinical and immunologic features in severe and moderate Coronavirus Disease 2019
- Record: found
- Abstract: found
- Article: not found
