- Record: found
- Abstract: found
- Article: found
Induction of Hsp70 in tumor cells treated with inhibitors of the Hsp90 activity: A predictive marker and promising target for radiosensitization
Read this article at
Abstract
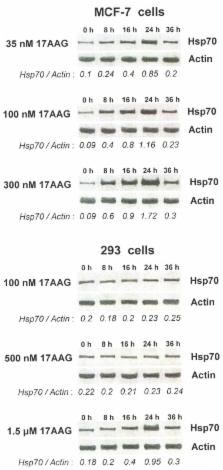
We studied a role of the inducible heat shock protein 70 (Hsp70) in cellular response to radiosensitizing treatments with inhibitors of the heat shock protein 90 (Hsp90) chaperone activity. Cell lines derived from solid tumors of different origin were treated with the Hsp90 inhibitors (17AAG, geldanamycin, radicicol, NVP-AUY922) or/and γ-photon radiation. For comparison, human cells of the non-cancerous origin were subjected to the same treatments. We found that the Hsp90 inhibitors yielded considerable radiosensitization only when they cause early and pronounced Hsp70 induction; moreover, a magnitude of radiosensitization was positively correlated with the level of Hsp70 induction. The quantification of Hsp70 levels in Hsp90 inhibitor-treated normal and cancer cells enabled to predict which of them will be susceptible to any Hsp90-inhibiting radiosensitizer as well as what concentrations of the inhibitors ensure the preferential cytotoxicity in the irradiated tumors without aggravating radiation damage to adjacent normal tissues. Importantly, the Hsp70 induction in the Hsp90 inhibitor-treated cancer cells appears to be their protective response that alleviates the tumor-sensitizing effects of the Hsp90 inactivation. Combination of the Hsp70-inducing inhibitors of Hsp90 with known inhibitors of the Hsp induction such as quercetin, triptolide, KNK437, NZ28 prevented up-regulation of Hsp70 in the cancer cells thereby increasing their post-radiation apoptotic/necrotic death and decreasing their post-radiation viability/clonogenicity. Similarly, co-treatment with the two inhibitors conferred the enhanced radiosensitization of proliferating rather than quiescent human vascular endothelial cells which may be used for suppressing the tumor-stimulated angiogenesis. Thus, the easily immunodetectable Hsp70 induction can be a useful marker for predicting effects of Hsp90-inhibiting radiosensitizers on tumors and normal tissues exposed to ionizing radiation. Moreover, targeting the Hsp70 induction in Hsp90 inhibitor-treated cancer cells and tumor vasculature cells may beneficially enhance the radiosensitizing effect.
Related collections
Most cited references32
- Record: found
- Abstract: found
- Article: not found
A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors.
- Record: found
- Abstract: found
- Article: not found
Protein folding in the cytoplasm and the heat shock response.
- Record: found
- Abstract: found
- Article: not found