- Record: found
- Abstract: found
- Article: found
Effectiveness and safety of eltrombopag in connective tissue disease patients with refractory immune thrombocytopenia: a retrospective study

Read this article at
Abstract
Objectives
We aimed to investigate the safety and effectiveness of eltrombopag for adult patients with refractory immune thrombocytopenia (ITP) secondary to connective tissue disease (CTD).
Methods
This is a single-centre, retrospective cohort and propensity score-matched study. Data from CTD-ITP patients treated with eltrombopag between January 2019 and January 2023 were retrospectively analysed. Baseline characteristics and follow-up information were recorded. CTD patients without ITP were matched to identify the risk factors associated with CTD-ITP performed by Logistic regression analysis.
Results
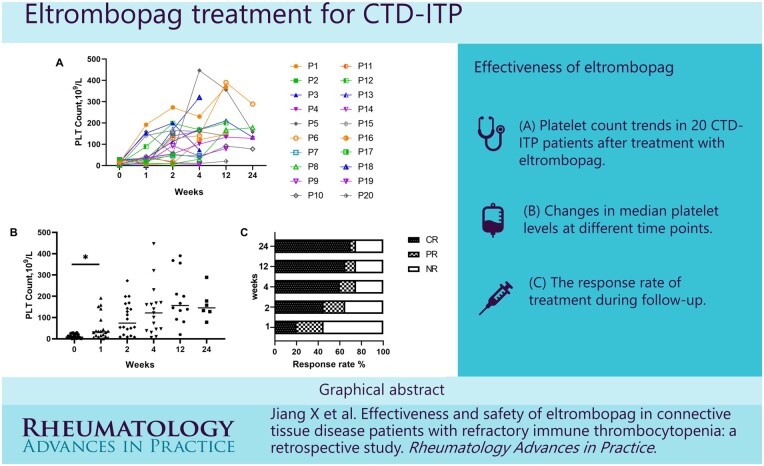
Twenty patients were enrolled, including 5 systemic lupus erythematosus (SLE), 9 Sjögren’s syndrome (SS) and 6 undifferentiated connective tissue disease (UCTD). Nineteen (95%) patients were female, and the median age was 59 years. Logistic regression analysis showed that anaemia (OR = 8.832, P = 0.007) was associated with increased risk of ITP, while non-erosive arthritis (OR = 0.045, P = 0.001) and interstitial lung disease (OR = 0.075, P = 0.031) were associated with reduced risk. Fourteen patients (70%) achieved a complete response (CR) and one (5%) achieved a partial response (PR). The median response time was 14 days. The median platelet count was 8.5 × 10 9/l at baseline of eltrombopag and increased to 122 × 10 9/l after 4 weeks. No adverse events were observed.
Graphical Abstract
Related collections
Most cited references20
- Record: found
- Abstract: found
- Article: not found
2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus
- Record: found
- Abstract: found
- Article: not found
Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group.
Author and article information
Comments
Comment on this article
 Smart Citations
Smart CitationsSee how this article has been cited at scite.ai
scite shows how a scientific paper has been cited by providing the context of the citation, a classification describing whether it supports, mentions, or contrasts the cited claim, and a label indicating in which section the citation was made.