- Record: found
- Abstract: found
- Article: found
Linc-RoR promotes MAPK/ERK signaling and confers estrogen-independent growth of breast cancer
Read this article at
Abstract
Background
The conversion from estrogen-dependent to estrogen-independent state of ER+ breast cancer cells is the key step to promote resistance to endocrine therapies. Although the crucial role of MAPK/ERK signaling pathway in estrogen-independent breast cancer cell growth is well established, the underlying mechanism is not fully understood.
Methods
In this study, we profiled lncRNA expression against a focused group of lncRNAs selected from lncRNA database. CRISPR/Cas9 was employed to knockout (KO) linc-RoR in MCF-7 cells, while rescue experiments were carried out to re-express linc-RoR in KO cells. Colony formation and MTT assays were used to examine the role of linc-RoR in estrogen-independent growth and tamoxifen resistance. Western blot and qRT-PCR were used to determine the change of protein and lncRNA levels, respectively. The expression of DUSP7 in clinical specimens was downloaded from Oncomine ( www.oncomine.org) and the dataset from Kaplan-Meier Plotter ( http://kmplot.com) was used to analyze the clinical outcomes in relation to DUSP7.
Results
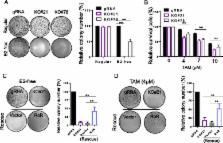
We identified that linc-RoR functions as an onco-lncRNA to promote estrogen-independent growth of ER+ breast cancer. Under estrogen deprivation, linc-RoR causes the upregulation of phosphorylated MAPK/ERK pathway which in turn activates ER signaling. Knockout of linc-RoR abrogates estrogen deprivation-induced ERK activation as well as ER phosphorylation, whereas re-expression of linc-RoR restores all above phenotypes. Moreover, we show that the ERK-specific phosphatase Dual Specificity Phosphatase 7 (DUSP7), also known as MKP-X, is involved in linc-RoR KO-induced repression of MAPK/ERK signaling. Interestingly, linc-RoR KO increases the protein stability of DUSP7, resulting in repression of ERK phosphorylation. Clinical data analysis reveal that DUSP7 expression is lower in ER+ breast cancer samples than that in ER- breast cancer. Moreover, downregulation of DUSP7 expression is associated with poor patient survival.
Conclusion
Taken together, these results suggest that linc-RoR promotes estrogen-independent growth and activation of MAPK/ERK pathway of breast cancer cells by regulating the ERK-specific phosphatase DUSP7. Thus, this study might help not only in establishing a role for linc-RoR in estrogen-independent and tamoxifen resistance of ER+ breast cancer, but also suggesting a link between linc-RoR and MAPK/ERK pathway.
Related collections
Most cited references35
- Record: found
- Abstract: found
- Article: not found
Efficient In Vivo Genome Editing Using RNA-Guided Nucleases
- Record: found
- Abstract: found
- Article: not found