- Record: found
- Abstract: found
- Article: found
Autophagy in endothelial cells and tumor angiogenesis

Read this article at
Abstract
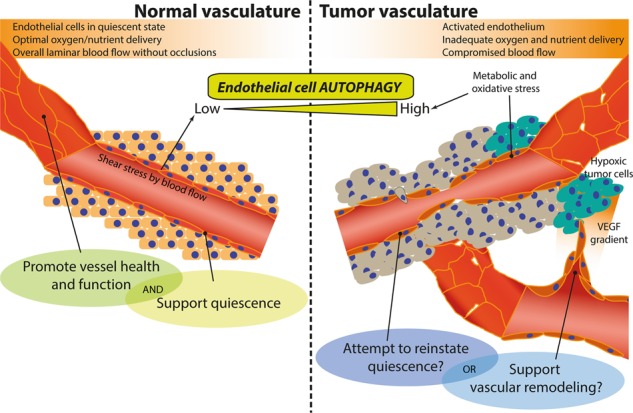
In mammalian cells, autophagy is the major pathway for the degradation and recycling of obsolete and potentially noxious cytoplasmic materials, including proteins, lipids, and whole organelles, through the lysosomes. Autophagy maintains cellular and tissue homeostasis and provides a mechanism to adapt to extracellular cues and metabolic stressors. Emerging evidence unravels a critical function of autophagy in endothelial cells (ECs), the major components of the blood vasculature, which delivers nutrients and oxygen to the parenchymal tissue. EC-intrinsic autophagy modulates the response of ECs to various metabolic stressors and has a fundamental role in redox homeostasis and EC plasticity. In recent years moreover, genetic evidence suggests that autophagy regulates pathological angiogenesis, a hallmark of solid tumors. In the hypoxic, nutrient-deprived, and pro-angiogenic tumor microenvironment, heightened autophagy in the blood vessels is emerging as a critical mechanism enabling ECs to dynamically accommodate their higher bioenergetics demands to the extracellular environment and connect with other components of the tumor stroma through paracrine signaling. In this review, we provide an overview of the major cellular mechanisms regulated by autophagy in ECs and discuss their potential role in tumor angiogenesis, tumor growth, and response to anticancer therapy.
Abstract
Vascular homeostasis relies on the proper behavior of endothelial cells (ECs). Emerging evidence indicate a critical role of autophagy, a vesicular process for lysosomal degradation of cytoplasmic content, in EC biology. While EC-intrinsic autophagy promotes EC function and quiescent state through redox homeostasis and possibly metabolic control, a role for EC-associated autophagy in cancer seems more complex.
Related collections
Most cited references74
- Record: found
- Abstract: found
- Article: not found
Molecular regulation of vessel maturation.
- Record: found
- Abstract: found
- Article: not found
Endocytosis and signalling: intertwining molecular networks.
- Record: found
- Abstract: found
- Article: not found