- Record: found
- Abstract: found
- Article: found
K-Means Clustering Identifies Diverse Clinical Phenotypes in COVID-19 Patients: Implications for Mortality Risks and Remdesivir Impact
Read this article at
Abstract
Introduction
The impact of remdesivir on mortality in patients with COVID-19 is still controversial. We aimed to identify clinical phenotype clusters of COVID-19 hospitalized patients with highest benefit from remdesivir use and validate these findings in an external cohort.
Methods
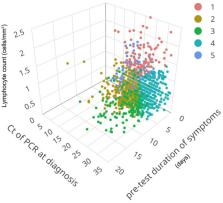
We included consecutive patients hospitalized between February 2020 and February 2021 for COVID-19. The derivation cohort comprised subjects admitted to Hospital Clinic of Barcelona. The validation cohort included patients from Hospital Universitari Mutua de Terrassa (Terrassa) and Hospital Universitari La Fe (Valencia), all tertiary centers in Spain. We employed K-means clustering to group patients according to reverse transcription polymerase chain reaction (rRT-PCR) cycle threshold (Ct) values and lymphocyte counts at diagnosis, and pre-test symptom duration. The impact of remdesivir on 60-day mortality in each cluster was assessed.
Results
A total of 1160 patients (median age 66, interquartile range (IQR) 55–78) were included. We identified five clusters, with mortality rates ranging from 0 to 36.7%. Highest mortality rate was observed in the cluster including patients with shorter pre-test symptom duration, lower lymphocyte counts, and lower Ct values at diagnosis. The absence of remdesivir administration was associated with worse outcome in the high-mortality cluster (10.5% vs. 36.7%; p < 0.001), comprising subjects with higher viral loads. These results were validated in an external multicenter cohort of 981 patients.
Related collections
Most cited references22
- Record: found
- Abstract: found
- Article: found
Remdesivir for the Treatment of Covid-19 — Final Report
- Record: found
- Abstract: found
- Article: not found
Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD): Explanation and Elaboration
- Record: found
- Abstract: found
- Article: not found