- Record: found
- Abstract: found
- Article: found
Solution and Film Self-Assembly Behavior of a Block Copolymer Composed of a Poly(ionic Liquid) and a Stimuli-Responsive Weak Polyelectrolyte
Read this article at
Abstract

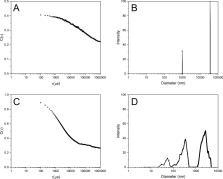
Cu(0)-mediated atom transfer radical polymerization was used to synthesize a poly(ionic liquid), poly[4-vinylbenzyl-3-butylimidazolium bis(trifluoromethylsulfonyl)imide] (PVBBImTf 2N), a stimuli-responsive polyelectrolyte, poly[2-(dimethylamino)ethyl methacrylate] (PDMAEMA), and a novel block copolymer formed from these two polymers. The synthesis of the block copolymer, poly[2-(dimethylamino) ethyl methacrylate]- block-[poly(4-vinylbenzyl-3-butylimidazolium bis(trifluoromethylsulfonyl)imide] (PDMAEMA- b-PVBBImTf 2N), was examined to evaluate the control of “livingness” polymerization, as indicated by molecular weight, characterizations of degree of polymerization, and 1HNMR spectroscopy. 2D DOSY NMR measurements revealed the successful formation of block copolymer and the connection between the two polymer blocks. PDMAEMA- b-PVBBImTf 2N was further characterized for supramolecular interactions in both the bulk and solution states through FTIR and 1H NMR spectroscopies. While the block copolymer demonstrated similar intermolecular behavior to the PIL homopolymer in the bulk state as indicated by FTIR, hydrogen bonding and counterion interactions in solution were observed in polar organic solvent through 1H NMR measurements. The DLS characterization revealed that the PDMAEMA- b-PVBBImTf 2N block copolymer forms a network-like aggregated structure due to a combination of hydrogen bonding between the PDMAEMA and PIL group and electrostatic repulsive interactions between PIL blocks. This structure was found to collapse upon the addition of KNO 3 while still maintaining hydrogen bonding interactions. AFM-IR analysis demonstrated varied morphologies, with spherical PDMAEMA in PVBBImTf 2N matrix morphology exhibited in the region approaching the film center. AFM-IR further revealed signals from silica nano-contaminates, which selectively interacted with the PDMAEMA spheres, demonstrating the potential for the PDMAEMA- b-PVBBImTf 2N PIL block copolymer in polymer–inorganic nanoparticle composite applications.
Related collections
Most cited references106
- Record: found
- Abstract: found
- Article: not found
Self-assembly of block copolymers.
- Record: found
- Abstract: not found
- Article: not found
Poly(ionic liquid)s: An update
- Record: found
- Abstract: not found
- Article: not found