- Record: found
- Abstract: found
- Article: found
Efficient Colon Cancer Immunogene Therapy Through Co-Delivery of IL-22BP mRNA and Tumor Cell Lysate by CLSV Nanoparticles
Read this article at
Abstract
Background
Messenger ribonucleic acid (mRNA)-based gene therapy has great potential in cancer treatment. However, the application of mRNA-based cancer treatment could be further developed. Elevated delivery ability and enhanced immune response are advantages for expanding the application of mRNA-based cancer therapy. It is crucial that the prepared carrier can cause an immune reaction based on the efficient delivery of mRNA.
Methods
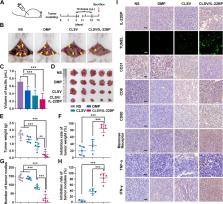
We reported DMP nanoparticle previously, which was obtained by the self-assembly of 1,2-dioleoyl-3-trimethylammonium propane (DOTAP) and (ethylene glycol)- b-poly (ε-caprolactone) (mPEG-PCL). Research demonstrated that DMP can deliver mRNA, siRNA, and plasmid. And it is applied to various tumor types. In our work, the tumor cell lysate was introduced to the internal DMP chain, fusing cell-penetrating peptides (CPPs) modification on the surface forming the CLSV system. And then mixed encoded IL-22BP (interleukin-22 binding protein) mRNA and CLSV to form CLSV/IL-22BP complex.
Results
The size of the CLSV system was 213.2 nm, and the potential was 45.7 mV. The transfection efficiency of the CLSV system is up to 76.45% in C26 cells via the micropinocytosis pathway. The CLSV system also could induce an immune response and significantly elevate the expression of CD80, CD86, and MHC-II in vivo. Then, by binding with IL-22BP (Interleukin-22 binding protein) mRNA, the CLSV/IL-22BP complex inhibited tumor cell growth, with an inhibition rate of up to 82.3% in vitro. The CLSV/IL-22BP complex also inhibited tumor growth in vivo, the tumor cell growth inhibition up to 75.0% in the subcutaneous tumor model, and 84.9% in the abdominal cavity metastasis tumor model.
Related collections
Most cited references38
- Record: found
- Abstract: found
- Article: not found
COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T-cell responses
- Record: found
- Abstract: found
- Article: not found
An immunogenic personal neoantigen vaccine for patients with melanoma
- Record: found
- Abstract: found
- Article: not found