- Record: found
- Abstract: found
- Article: found
Drinking Molecular Hydrogen Water Is Beneficial to Cardiovascular Function in Diet-Induced Obesity Mice
Read this article at
Abstract
Simple Summary
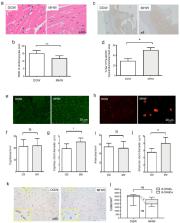
Molecular hydrogen (MH) reportedly exerts therapeutic effects against inflammatory diseases by alleviating oxidative stress. We investigated the cardiovascular protective effects of molecular hydrogen water (MHW) intake using high-fat diet-induced obesity (DIO) mice. We observed that MHW intake for 2 weeks did not improve the blood sugar level or body weight but decreased heart weight in DIO mice. Notably, MHW intake alleviated oxidative stress in both the heart and the adipose tissue. Moreover, it improved cardiac hypertrophy and restored left ventricular function in DIO mice, and promoted the histological conversion of energy storage to expenditure in adipose tissues with the upregulation of thermogenic and cardiovascular protective genes. Furthermore, MHW restored endothelial progenitor cell (EPC) bioactivity to maintain vascular homeostasis. Taken together, MHW intake exerts cardiovascular protective effects in DIO mice. Hence, MHW intake is a potential prophylactic strategy against cardiovascular disorders in metabolic syndrome.
Abstract
Molecular hydrogen (MH) reportedly exerts therapeutic effects against inflammatory diseases as a suppressor of free radical chain reactions. Here, the cardiovascular protective effects of the intake of molecular hydrogen water (MHW) were investigated using high-fat diet-induced obesity (DIO) mice. MHW was prepared using supplier sticks and degassed water as control. MHW intake for 2 weeks did not improve blood sugar or body weight but decreased heart weight in DIO mice. Moreover, MHW intake improved cardiac hypertrophy, shortened the width of cardiomyocytes, dilated the capillaries and arterioles, activated myocardial eNOS-Ser-1177 phosphorylation, and restored left ventricular function in DIO mice. MHW intake promoted the histological conversion of hypertrophy to hyperplasia in white and brown adipose tissues (WAT and BAT) with the upregulation of thermogenic and cardiovascular protective genes in BAT (i.e., Ucp-1, Vegf-a, and eNos). Furthermore, the results of a colony formation assay of bone-marrow-derived endothelial progenitor cells (EPCs) indicated that MHW activated the expansion, differentiation, and mobilization of EPCs to maintain vascular homeostasis. These findings indicate that the intake of MHW exerts cardiovascular protective effects in DIO mice. Hence, drinking MHW is a potential prophylactic strategy against cardiovascular disorders in metabolic syndrome.
Related collections
Most cited references52
- Record: found
- Abstract: found
- Article: not found
Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study.
- Record: found
- Abstract: found
- Article: not found
Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human.
- Record: found
- Abstract: found
- Article: not found