- Record: found
- Abstract: found
- Article: found
Dapagliflozin Alleviates Hepatic Steatosis by Restoring Autophagy via the AMPK-mTOR Pathway
Read this article at
Abstract
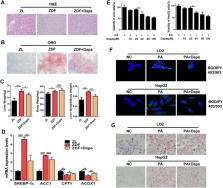
As a newly approved oral hypoglycaemic agent, the sodium-glucose cotransporter 2 (SGLT2) inhibitor dapagliflozin, which is derived from the natural product phlorizin can effectively reduce blood glucose. Recent clinical studies have found that dapagliflozin alleviates non-alcoholic fatty liver disease (NAFLD), but the specific mechanism remains to be explored. This study aimed to investigate the underlying mechanism of dapagliflozin in alleviating hepatocyte steatosis in vitro and in vivo. We fed the spontaneous type 2 diabetes mellitus rats with high-fat diets and cultured human normal liver LO2 cells and human hepatocellular carcinoma HepG2 cells with palmitic acid (PA) to induce hepatocellular steatosis. Dapagliflozin attenuated hepatic lipid accumulation both in vitro and in vivo. In Zucker diabetic fatty (ZDF) rats, dapagliflozin reduced hepatic lipid accumulation via promoting phosphorylation of acetyl-CoA carboxylase 1 (ACC1), and upregulating lipid β-oxidation enzyme acyl-CoA oxidase 1 (ACOX1). Furthermore, dapagliflozin increased the expression of the autophagy-related markers LC3B and Beclin1, in parallel with a drop in p62 level. Similar effects were observed in PA-stimulated LO2 cells and HepG2 cells. Dapagliflozin treatment could also significantly activated AMPK and reduced the phosphorylation of mTOR in ZDF rats and PA-stimulated LO2 cells and HepG2 cells. We demonstrated that dapagliflozin ameliorates hepatic steatosis by decreasing lipogenic enzyme, while inducing fatty acid oxidation enzyme and autophagy, which could be associated with AMPK activation. Moreover, our results indicate that dapagliflozin induces autophagy via the AMPK-mTOR pathway. These findings reveal a novel clinical application and functional mechanism of dapagliflozin in the treatment of NAFLD.
Related collections
Most cited references36
- Record: found
- Abstract: found
- Article: not found
AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1.
- Record: found
- Abstract: found
- Article: not found
Role of AMP-activated protein kinase in mechanism of metformin action.
- Record: found
- Abstract: found
- Article: not found