- Record: found
- Abstract: found
- Article: found
Complete remission of advanced pancreatic cancer induced by claudin18.2-targeted CAR-T cell therapy: a case report
Read this article at
Abstract
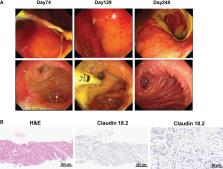
Pancreatic cancer (PC) is one of the most malignant tumors in digestive system due to its highly invasive and metastatic properties. At present, conventional treatment strategies for PC show the limited clinical efficacy. Therefore, novel effective therapeutic strategies are urgently needed. Here, we report a case of complete remission of advanced PC induced by claudin18.2-targeted CAR-T cell therapy. The patient was a 72-year-old man who was diagnosed with pancreatic ductal adenocarcinoma 2 years ago, and he experienced tumor recurrence and multiple metastases after pancreaticoduodenectomy and multi-line chemotherapies, including liver, peritoneum, and cervical lymph node metastases. Then, the patient was referred to our department for further treatment of metastatic PC, and he was enrolled in a clinical trial of claudin18.2-targeted CAR-T cell therapy. After lymphodepleting chemotherapy, the patient received claudin18.2-targeted CAR-T cell infusion at a dose of 1.2 × 10 6 cells/kg on November 21, 2022. During CAR-T cell therapy, the patient experienced grade 2 cytokine release syndrome (CRS) and gastric mucosa injury, which were controlled by tocilizumab and conventional symptomatic and supportive treatment. The patient achieved a complete response (CR) 1 month after claudin18.2-targeted CAR-T cell therapy, and remained in clinical remission for 8 months. Unfortunately, the patient experienced claudin18.2-negative relapse in July, 2023. Despite antigen-negative relapse after claudin18.2-targeted CAR-T cell infusion, the patient achieved sustained remission for 8 months, which indicates that claudin18.2-targeted CAR-T cell therapy is an extremely effective therapeutic strategy for the treatment of advanced PC.
Related collections
Most cited references23
- Record: found
- Abstract: found
- Article: not found
Pancreatic Cancer : A Review
- Record: found
- Abstract: found
- Article: not found
Distant Metastasis Occurs Late during the Genetic Evolution of Pancreatic Cancer
- Record: found
- Abstract: found
- Article: not found