- Record: found
- Abstract: found
- Article: found
The Ketamine Antidepressant Story: New Insights
Read this article at
Abstract
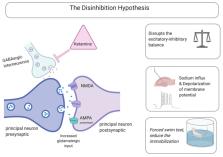
Ketamine is a versatile agent primarily utilized as a dissociative anesthetic, which acts by blocking the excitatory receptor N-methyl- d-aspartate receptor (NMDA). It functions to inhibit the current of both Na + and K + voltage-gated channels, thus preventing serotonin and dopamine reuptake. Studies have indicated that administering a single subanesthetic dose of ketamine relieves depression rapidly and that the effect is sustained. For decades antidepressant agents were based on the monoamine theory. Although ketamine may not be the golden antidepressant, it has opened new avenues toward mechanisms involved in the pathology of treatment-resistant depression and achieving rapid antidepressant effects. Thus, preclinical studies focusing on deciphering the molecular mechanisms involved in the antidepressant action of ketamine will assist in the development of a new antidepressant. This review was conducted to elucidate the emerging pathways that can explain the complex dose-dependent mechanisms achieved by administering ketamine to treat major depressive disorders. Special attention was paid to reviewing the literature on hydroxynorketamines, which are ketamine metabolites that have recently attracted attention in the context of depression.
Related collections
Most cited references105
- Record: found
- Abstract: found
- Article: not found
NMDA Receptor Blockade at Rest Triggers Rapid Behavioural Antidepressant Responses
- Record: found
- Abstract: found
- Article: not found
mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists.
- Record: found
- Abstract: found
- Article: not found