- Record: found
- Abstract: found
- Article: found
Cell Hashing with barcoded antibodies enables multiplexing and doublet detection for single cell genomics
Read this article at
Abstract
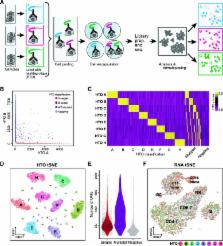
Despite rapid developments in single cell sequencing, sample-specific batch effects, detection of cell multiplets, and experimental costs remain outstanding challenges. Here, we introduce Cell Hashing, where oligo-tagged antibodies against ubiquitously expressed surface proteins uniquely label cells from distinct samples, which can be subsequently pooled. By sequencing these tags alongside the cellular transcriptome, we can assign each cell to its original sample, robustly identify cross-sample multiplets, and “super-load” commercial droplet-based systems for significant cost reduction. We validate our approach using a complementary genetic approach and demonstrate how hashing can generalize the benefits of single cell multiplexing to diverse samples and experimental designs.
Related collections
Most cited references16
- Record: found
- Abstract: found
- Article: not found
OUP accepted manuscript
- Record: found
- Abstract: found
- Article: not found