- Record: found
- Abstract: found
- Article: found
Quantitative proteomics reveals the protective effects of Yinchenzhufu decoction against cholestatic liver fibrosis in mice by inhibiting the PDGFRβ/PI3K/AKT pathway
Read this article at
Abstract
Introduction: Yinchenzhufu decoction (YCZFD) is a traditional Chinese medicine formula with hepatoprotective effects. In this study, the protective effects of YCZFD against cholestatic liver fibrosis (CLF) and its underlying mechanisms were evaluated.
Methods: A 3, 5-diethoxycarbonyl-1, 4-dihydro-collidine (DDC)-induced cholestatic mouse model was used to investigate the amelioration of YCZFD on CLF. Data-independent acquisition-based mass spectrometry was performed to investigate proteomic changes in the livers of mice in three groups: control, model, and model treated with high-dose YCZFD. The effects of YCZFD on the expression of key proteins were confirmed in mice and cell models.
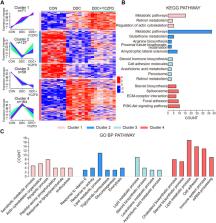
Results: YCZFD significantly decreased the levels of serum biochemical, liver injury, and fibrosis indicators of cholestatic mice. The proteomics indicated that 460 differentially expressed proteins (DEPs) were identified among control, model, and model treated with high-dose YCZFD groups. Enrichment analyses of these DEPs revealed that YCZFD influenced multiple pathways, including PI3K-Akt, focal adhesion, ECM–receptor interaction, glutathione metabolism, and steroid biosynthesis pathways. The expression of platelet derived growth factor receptor beta (PDGFRβ), a receptor associated with the PI3K/AKT and focal adhesion pathways, was upregulated in the livers of cholestatic mice but downregulated by YCZFD. The effects of YCZFD on the expression of key proteins in the PDGFRβ/PI3K/AKT pathway were further confirmed in mice and transforming growth factor-β-induced hepatic stellate cells. We uncovered seven plant metabolites (chlorogenic acid, scoparone, isoliquiritigenin, glycyrrhetinic acid, formononetin, atractylenolide I, and benzoylaconitine) of YCZFD that may regulate PDGFRβ expression.
Conclusion: YCZFD substantially protects against DDC-induced CLF mainly through regulating the PDGFRβ/PI3K/AKT signaling pathway.
Related collections
Most cited references44
- Record: found
- Abstract: found
- Article: not found
Universal sample preparation method for proteome analysis.
- Record: found
- Abstract: found
- Article: not found
Molecular and cellular mechanisms of liver fibrosis and its regression
- Record: found
- Abstract: found
- Article: not found