- Record: found
- Abstract: found
- Article: found
Incidence of CXCR4 tropism and CCR5-tropic resistance in treatment-experienced participants receiving maraviroc in the 48-week MOTIVATE 1 and 2 trials
Read this article at
Abstract
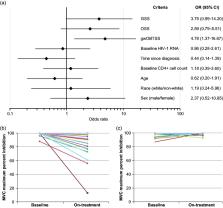
Maraviroc blocks HIV-1 entry into CD4+ cells by interrupting the interaction between viral gp120 and cell-surface CCR5. Resistance to CCR5 antagonist–mediated inhibition can develop by unmasking pre-existing CXCR4-using virus or through selection of CCR5-tropic resistant virus, characterized by plateaus in maximum percent inhibition <95%. Here, we examine viral escape in maraviroc-treated participants during virologic failure through Week 48 in the MOTIVATE 1 and 2 trials. Resistance was assessed relative to number of active drugs in participants’ optimized background therapy, pharmacokinetic adherence markers, Baseline demographic data, HIV-1 RNA and CD4+ counts. For participants with R5 virus confirmed ( post hoc) at Screening, Baseline genotypic weighted optimized background therapy susceptibility scores (gwOBTSS) were assigned where possible. Through Week 48, 219/392 (56%) participants with an assigned gwOBTSS achieved a virologic response. Of those remaining, 48/392 (12%) had CXCR4-using virus; 58/392 (15%) had R5 virus (maraviroc sensitive: n = 35/392, 9%; maraviroc resistant: n = 18/392, 5%; undeterminable: n = 5/392, 1%) and 67/392 (17%) had no failure tropism result. When optimized background therapy provided limited support to maraviroc (gwOBTSS <2), 143/286 (50%) responded to therapy, while 76/106 (72%) participants with gwOBTSS ≥2 responded ( p < 0.001). Resistance rates were highest for participants with gwOBTSS <2, accounting for 45/48 (94%) of total CXCR4-using emergence and 18/18 (100%) of total CCR5-tropic resistance. R5 viruses from participants with gwOBTSS ≥2 ( n = 10) were exclusively maraviroc sensitive; five of these participants had pharmacokinetic and/or pill-count markers of non-adherence. When co-administered with a fully active background regimen, maraviroc did not readily generate resistance in the clinical setting.
Related collections
Most cited references22
- Record: found
- Abstract: found
- Article: not found
Maraviroc (UK-427,857), a potent, orally bioavailable, and selective small-molecule inhibitor of chemokine receptor CCR5 with broad-spectrum anti-human immunodeficiency virus type 1 activity.
- Record: found
- Abstract: found
- Article: not found
Structure of the CCR5 chemokine receptor-HIV entry inhibitor maraviroc complex.
- Record: found
- Abstract: found
- Article: not found