- Record: found
- Abstract: found
- Article: found
Mechanism and therapeutic effect of umbilical cord mesenchymal stem cells in inflammatory bowel disease

Read this article at
Abstract
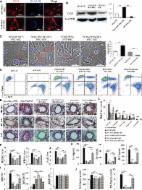
Inflammatory bowel disease (IBD) is a persistent and chronic disease that is characterized by destructive gastrointestinal (GI) inflammation. Researchers are trying to identify and develop new and more effective treatments with no side effects. Acute and chronic mouse models of IBD were established using dextran sulfate sodium (DSS) solution. To evaluate the efficacy and mechanism, umbilical cord mesenchymal stem cells (UCMSCs) were obtained from Kunming (KM) mice and humans. In the chronic IBD study, the survival rates of the normal control, model, mouse UCMSC (mUCMSC) and human UCMSC (hUCMSC) groups were 100%, 40%, 86.7%, and 100%, respectively. The histopathological scores of the normal control, intraperitoneal injection, intravenous treatment, and model groups were 0.5 ± 0.30, 5.9 ± 1.10, 8.7 ± 1.39, and 8.8 ± 1.33 (p = 0.021). UCMSCs promoted the expression of the intestinal tight junction protein occludin, downregulated the protein expression of the autophagy marker LC3A/B in colon tissue, and upregulated the expression of VEGF-A and VEGFR-1 at the injured site. This study provides an experimental model for elucidating the therapeutic effects of UCMSCs in IBD. We provide a theoretical basis and method for the clinical treatment of IBD using UCMSCs.
Related collections
Most cited references29
- Record: found
- Abstract: found
- Article: not found
Human umbilical cord matrix stem cells: preliminary characterization and effect of transplantation in a rodent model of Parkinson's disease.
- Record: found
- Abstract: found
- Article: not found
Mesenchymal stromal cell 'licensing': a multistep process.
- Record: found
- Abstract: found
- Article: found