- Record: found
- Abstract: found
- Article: not found
Feasibility of preoperative chemotherapy for locally advanced, operable colon cancer: the pilot phase of a randomised controlled trial
Read this article at
Summary
Background
Preoperative (neoadjuvant) chemotherapy and radiotherapy are more effective than similar postoperative treatment for oesophageal, gastric, and rectal cancers, perhaps because of more effective micrometastasis eradication and reduced risk of incomplete excision and tumour cell shedding during surgery. The FOxTROT trial aims to investigate the feasibility, safety, and efficacy of preoperative chemotherapy for colon cancer.
Methods
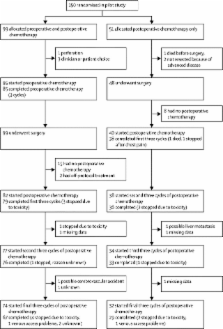
In the pilot stage of this randomised controlled trial, 150 patients with radiologically staged locally advanced (T3 with ≥5 mm invasion beyond the muscularis propria or T4) tumours from 35 UK centres were randomly assigned (2:1) to preoperative (three cycles of OxMdG [oxaliplatin 85 mg/m 2, l-folinic acid 175 mg, fluorouracil 400 mg/m 2 bolus, then 2400 mg/m 2 by 46 h infusion] repeated at 2-weekly intervals followed by surgery and a further nine cycles of OxMdG) or standard postoperative chemotherapy (12 cycles of OxMdG). Patients with KRAS wild-type tumours were randomly assigned (1:1) to receive panitumumab (6 mg/kg; every 2 weeks with the first 6 weeks of chemotherapy) or not. Treatment allocation was through a central randomisation service using a minimised randomisation procedure including age, radiological T and N stage, site of tumour, and presence of defunctioning colostomy as stratification variables. Primary outcome measures of the pilot phase were feasibility, safety, and tolerance of preoperative therapy, and accuracy of radiological staging. Analysis was by intention to treat. This trial is registered, number ISRCTN 87163246.
Findings
96% (95 of 99) of patients started and 89% (85 of 95) completed preoperative chemotherapy with grade 3–4 gastrointestinal toxicity in 7% (seven of 94) of patients. All 99 tumours in the preoperative group were resected, with no significant differences in postoperative morbidity between the preoperative and control groups: 14% (14 of 99) versus 12% (six of 51) had complications prolonging hospital stay (p=0·81). 98% (50 of 51) of postoperative chemotherapy patients had T3 or more advanced tumours confirmed at post-resection pathology compared with 91% (90 of 99) of patients following preoperative chemotherapy (p=0·10). Preoperative therapy resulted in significant downstaging of TNM5 compared with the postoperative group (p=0·04), including two pathological complete responses, apical node involvement (1% [one of 98] vs 20% [ten of 50], p<0·0001), resection margin involvement (4% [four of 99] vs 20% [ten of 50], p=0·002), and blinded centrally scored tumour regression grading: 31% (29 of 94) vs 2% (one of 46) moderate or greater regression (p=0·0001).
Interpretation
Preoperative chemotherapy for radiologically staged, locally advanced operable primary colon cancer is feasible with acceptable toxicity and perioperative morbidity. Proceeding to the phase 3 trial, to establish whether the encouraging pathological responses seen with preoperative therapy translates into improved long-term oncological outcome, is appropriate.
Related collections
Most cited references23
- Record: found
- Abstract: found
- Article: not found
Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer.
- Record: found
- Abstract: found
- Article: not found
Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial.
- Record: found
- Abstract: found
- Article: not found
