- Record: found
- Abstract: found
- Article: found
β1 integrin-mediated multicellular resistance in hepatocellular carcinoma through activation of the FAK/Akt pathway

Read this article at
Abstract
Objective
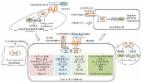
To explore the role and mechanism of β1 integrin in the regulation of multicellular drug resistance in hepatocellular carcinoma (HCC).
Methods
This in vitro study used a liquid overlay technique to obtain multicellular spheroids of two human HCC cell lines, HepG2 and Bel-7402. The morphology of the spheroids was observed by optical and electron microscopy. The effects of exposure to 5-fluorouracil (5-FU) and cisplatin (CDDP) on cell proliferation and the induction of apoptosis were assessed in monolayer cells and multicellular spheroids. The levels of β1 integrin and the effects on the focal adhesion kinase (FAK)/protein kinase B (Akt) pathway were evaluated using Western blot analysis, immunofluorescence and flow cytometry. The role of β1 integrin was confirmed by using an inhibitory antibody.
Results
Cell proliferation inhibition and cell apoptosis induced by 5-FUl and CDDP were abrogated in multicellular spheroids compared with monolayer cells. There were high levels of β1 integrin in multicellular spheroids. β1 integrin inhibitory antibody prevented the formation of multicellular spheroids, coupled with a significant increase in proliferation inhibition and apoptosis induction. β1 integrin inhibitory antibody effectively suppressed activation of both FAK and Akt in multicellular spheroids.
Related collections
Most cited references31
- Record: found
- Abstract: found
- Article: not found
Focal adhesion signaling and therapy resistance in cancer.
- Record: found
- Abstract: found
- Article: not found
AKT in cancer: new molecular insights and advances in drug development.
- Record: found
- Abstract: found
- Article: found