- Record: found
- Abstract: found
- Article: found
Progress of oncolytic virotherapy for neuroblastoma
Read this article at
Abstract
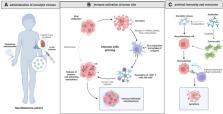
As a neuroendocrine tumor derived from the neural crest, neuroblastoma (NB) is the most common extracranial solid tumor in children. The prognosis in patients with low- and intermediate-risk NB is favorable while that in high-risk patients is often detrimental. However, the management of the considerably large proportion of high-risk patients remains challenging in clinical practice. Among various new approaches, oncolytic virus (OV) therapy offers great advantages in tumor treatment, especially for high-risk NB. Genetic modified OVs can target NB specifically without affecting normal tissue and avoid the widespread drug resistance issue in anticancer monotherapy. Meanwhile, its safety profile provides great potential in combination therapy with chemo-, radio-, and immunotherapy. The therapeutic efficacy of OV for NB is impressive from bench to bedside. The effectiveness and safety of OVs have been demonstrated and reported in studies on children with NB. Furthermore, clinical trials on some OVs (Celyvir, Pexa-Vec (JX-594) and Seneca Valley Virus (NTX-010)) have reported great results. This review summarizes the latest evidence in the therapeutic application of OVs in NB, including those generated in cell lines, animal models and clinical trials.
Related collections
Most cited references138
- Record: found
- Abstract: not found
- Article: not found
Recent advances in neuroblastoma.
- Record: found
- Abstract: found
- Article: not found