- Record: found
- Abstract: found
- Article: found
Blood donor exposome and impact of common drugs on red blood cell metabolism

Read this article at
Abstract
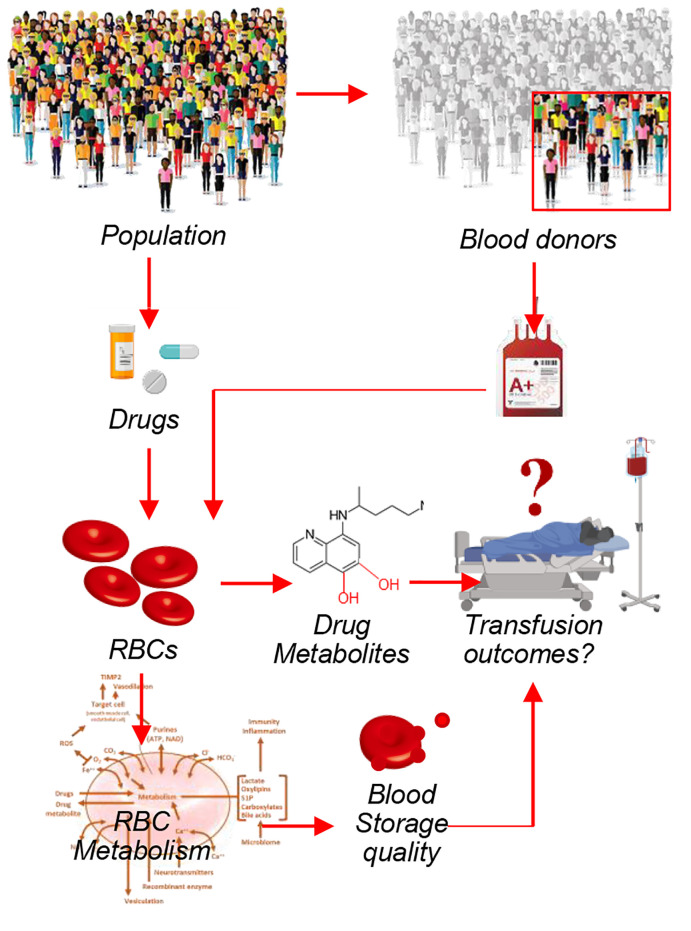
Computational models based on recent maps of the RBC proteome suggest that mature erythrocytes may harbor targets for common drugs. This prediction is relevant to RBC storage in the blood bank, in which the impact of small molecule drugs or other xenometabolites deriving from dietary, iatrogenic, or environmental exposures (“exposome”) may alter erythrocyte energy and redox metabolism and, in so doing, affect red cell storage quality and posttransfusion efficacy. To test this prediction, here we provide a comprehensive characterization of the blood donor exposome, including the detection of common prescription and over-the-counter drugs in blood units donated by 250 healthy volunteers in the Recipient Epidemiology and Donor Evaluation Study III Red Blood Cell–Omics (REDS-III RBC-Omics) Study. Based on high-throughput drug screenings of 1366 FDA-approved drugs, we report that approximately 65% of the tested drugs had an impact on erythrocyte metabolism. Machine learning models built using metabolites as predictors were able to accurately predict drugs for several drug classes/targets (bisphosphonates, anticholinergics, calcium channel blockers, adrenergics, proton pump inhibitors, antimetabolites, selective serotonin reuptake inhibitors, and mTOR), suggesting that these drugs have a direct, conserved, and substantial impact on erythrocyte metabolism. As a proof of principle, here we show that the antacid ranitidine — though rarely detected in the blood donor population — has a strong effect on RBC markers of storage quality in vitro. We thus show that supplementation of blood units stored in bags with ranitidine could — through mechanisms involving sphingosine 1–phosphate–dependent modulation of erythrocyte glycolysis and/or direct binding to hemoglobin — improve erythrocyte metabolism and storage quality.
Abstract
Related collections
Most cited references50
- Record: found
- Abstract: found
- Article: not found
An estimation of the number of cells in the human body.
- Record: found
- Abstract: found
- Article: not found
Red blood cell storage lesion: causes and potential clinical consequences
- Record: found
- Abstract: found
- Article: not found